- Title
-
Mutations affecting the formation of the notochord in the zebrafish, Danio rerio
- Authors
- Odenthal, J., Haffter, P., Vogelsang, E., Brand, M., van Eeden, F.J., Furutani-Seiki, M., Granato, M., Hammerschmidt, M., Heisenberg, C.P., Jiang, Y.J., Kane, D.A., Kelsh, R.N., Mullins, M.C., Warga, R.M., Allende, M.L., Weinberg, E.S., and Nüsslein-Volhard, C.
- Source
- Full text @ Development
|
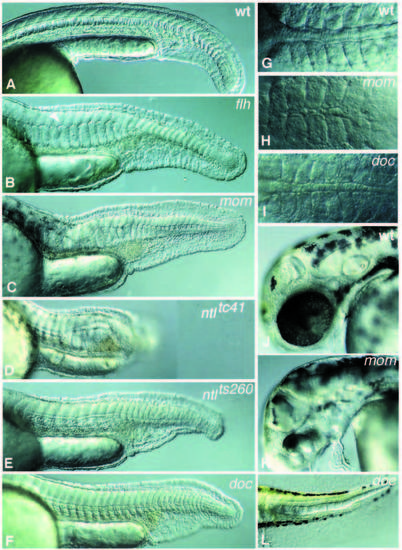
Images of live mutant embryos. Anterior is left, dorsal is top in all pictures, except in G-I, which are dorsal views. (A) 24-hour old wild-type embryo. (B) flhtk241 embryo; a small patch of floor plate in the ventral neural tube is indicated by an arrowhead. (C) momth211 embryo. (D) ntl embryo with the strongest allele, ntltc41. (E) ntlts260, the weak allele produces a reduced ventral tailfin. (F) doctt258 embryo. (G-I) Dorsal view of 10-somite stage embryos: (G) wild type, (H) momth211 and (I) doctt258. (J-K) Heads of 48-hour (J) wild-type and (K) mom larvae in a lateral view. (L) Tailtip of a 60 hour doctt258 larva. PHENOTYPE:
|
|
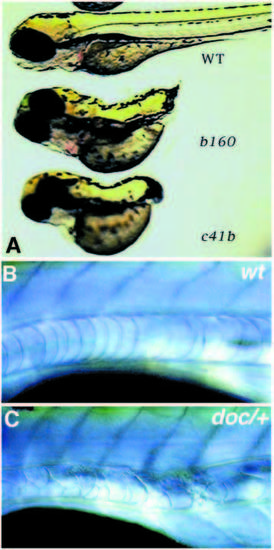
(A) 3-day old wild-type, ntlb160 and ntltc41 larvae. (B,C) The anterior notochord in (B) a wild-type and (C) a heterozygous doc/+ larva after 5 days of development. PHENOTYPE:
|
|
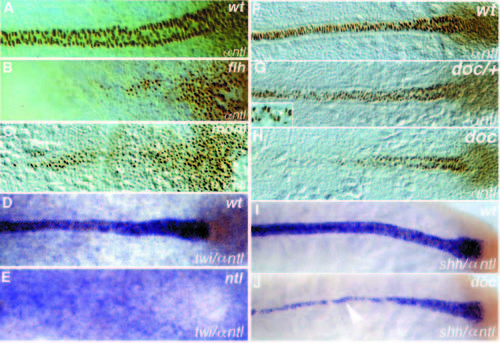
Dorsal views of (A-E) tailbud stage and (F-J) 10-somite stage embryos. (A-C, F-H) Whole-mount Ntl antibody staining; (D,E) twi in situ hybridization (blue) and Ntl antibody staining (brown); (I, J) shh in situ hybridization (blue) and Ntl antibody staining (brown). Anterior is to the left. (A,D,F,I) Wild type, (B) flhtk241, (C) momth211, (E) ntltc41, (G) heterozygous and (H, J) homozygous doctt258 embryos. Ntl expression in the axial mesoderm is reduced in (B) flh and (C) mom embryos at the tailbud stage. Expression of twi in (D) wild-type and (E) ntltc41 embryos, note that expression in the notochord is not detectable. (G) In heterozygous doctt258/+ embryos, Ntl is not detectable in a few nuclei of the notochord (arrowhead in the enlargement). In (J) doctt258 embryos notochord expression of shh (blue) is only detectable in notochord cells which also express Ntl (brown, compare to Ntl expression in H). In regions to the left of the arrowhead (anterior), shh expression is restricted to the floor plate. EXPRESSION / LABELING:
|
|
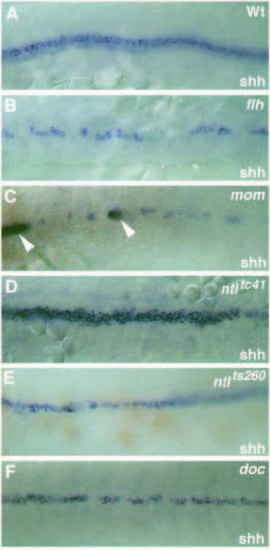
Dorsal view (anterior to the left) of the expression pattern of the floor plate marker shh at the 15-somite stage in (A) wild-type, (B) flhtk241, (C) momth211, (D) ntltc41, (E) ntlts260 and (F) doctt258 embryos. (A) At the 15-somite stage shh is expressed in the notochord (weak, out of focus) and in the midline floor plate cell of the spinal cord (strong). (C) In momth211 embryos shh is expressed in a few faintly labeled cells in the ventral spinal cord. These cells frequently overlie cells in the dorsal mesoderm expressing both shh and ntl (arrowheads, out of focus). (D) Note that in embryos with the strong allele, ntltc41, no expression of shh in the axial mesoderm is detectable. (E) Axial mesodermal expression of shh is strongly reduced in mutant embryos with the weak allele, ntlts260. EXPRESSION / LABELING:
|
|
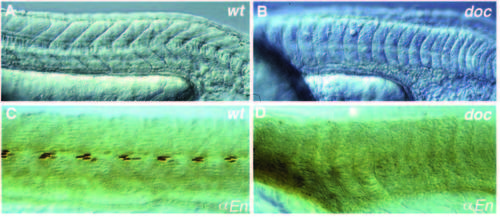
Formation of the horizontal myoseptum is affected in doctt258 embryos. (A) Somites of a wild-type embryo showing the characteristic vshape at 24 hours of development. (B) In doctt258 embryos, as in flh, mom and ntl embryos, the somites are u-shaped instead of v-shaped. (C) In the apex of the v, the nuclei of muscle pioneer cells stain with the antibody against Eng. (D) Eng staining in the somites is not detectable in doctt258 embryos, whereas expression in the midbrain-hindbrain boundary is normal (not shown). Lateral views, anterior is to the left. PHENOTYPE:
|
|
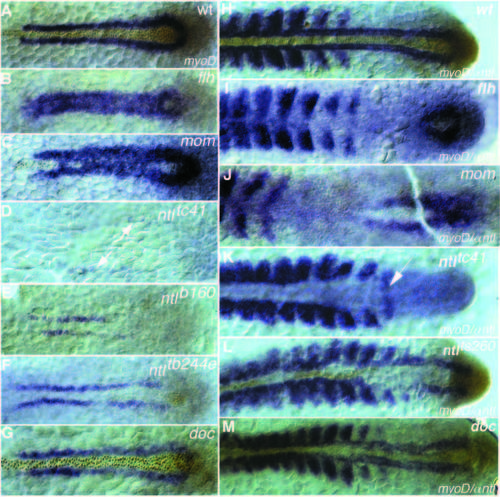
Dorsal view of myoD RNA (blue staining) and Ntl antibody staining (brown nuclear staining) at (A-G) tailbud stage and (H-M) 15- somite stage of (A,H) wild-type, (B,I) flhtk241, (C,J) momth211, (D,K) ntltc41, (E) ntlb160, (F) ntltb244e, (L) ntlts260 and (G,M) doctt258 embryos. (A) In wild type at the tailbud stage myoD expression is restricted to a line of cells left and right of the notochord. (B,C) The gap between the two stripes is filled with myoD expressing cells in (B) flhtk241 and (C) momth211 embryos. ntl expression is detectable only in the tailbud and sometimes in a few cells anteriorly, which do not express myoD (C). (D) Strongly reduced myoD expression a greater distance from the midline is detectable in embryos with the strong allele, ntltc41(arrows). myoD expression at the 15-somite stage in (H) wild type is expressed in the adaxial cells and in the posterior part of the somites. In (I) flhtk241 embryos and (J) momth211 embryos myoD expression in the adaxial cells is not maintained. (K) In embryos with the strong allele, ntltc41, somites are separated by a big gap of nonexpressing cells, whereas the 15th somite is fused in the midline (arrow). (L) In embryos with the weak allele, ntlts260, adaxial cells next to ntl-expressing cells express myoD at a higher level than the somites on the opposite side. (M) In doctt258 embryos adaxial expression in anterior regions is reduced and closer together. EXPRESSION / LABELING:
|
|
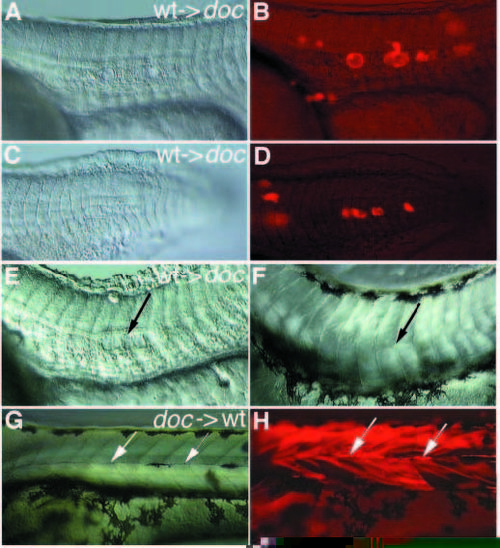
(A,C,E,F,G) Nomarski optics and (B,D,H) fluorescence pictures of transplantation experiments performed in doctt258 mutant embryos. Lateral view, anterior to the left. (A-D) Only wild-type derived donor cells in a mutant environment give rise to vacuolated notochord cells. Two different experiments are shown (A,B and C,D). (E) Wild-type cells in a mutant environment at 24 hours of development induce a horizontal myoseptum in some neighboring somites on day 3 (arrow in F). Mutant donor cells in wild-type somites give rise to normal horizontal myosepta (arrows in G,H), which can be identified by their strong fluorescence. In some regions this strong fluorescence is quenched by melanophores populating the horizontal myoseptum. |
|
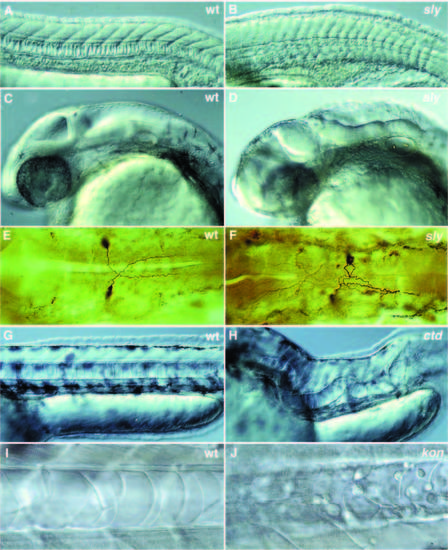
Photographs of a variety of selected mutants with late notochord defects. (A-D) Lateral views of 1- day old wild-type and mutant zebrafish embryos. (A,C) 1-day old wild-type embryo. (B,D) 1-day old slyti263a. (A) In wild type, the notochord cells are vacuolated and the notochord has a ‘stack-of-pennies’ appearance. (B) In embryos with strong alleles of sly, the notochord is thinner and poorly vacuolated. In a lateral view, the brain of sly embryos looks very disorganized when compared to wild type (C,D). (E,F) Antibody staining of the Mauthner neurons in 1-day old embryos using the monoclonal antibody 3A10. (E) In wild type, the axons of the Mauthner neurons cross the midline and grow towards the posterior. (F) In sly, the axons of one or both Mauthner neurons frequently fail to cross the midline and grow posteriorly along the ipsilateral side. (G,H) Lateral views of 2-day old wild-type and mutant embryos. (G) Lateral view of wild type with a normal, straight notochord. (H) Lateral view of ctd, in which the notochord extends in a random fashion throughout the embryo. (I) Notochord of a 40-hour old wildtype embryo with an intact notochord sheath. (J) Notochord of a 40-hour old kontc230 embryo. The vacuolated notochord cells are surrounded by undifferentiated round cells that failed to become notochord sheath cells. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|