- Title
-
Developmental independence of median fins from the larval fin fold revises their evolutionary origin
- Authors
- Miyamoto, K., Kawakami, K., Tamura, K., Abe, G.
- Source
- Full text @ Sci. Rep.
|
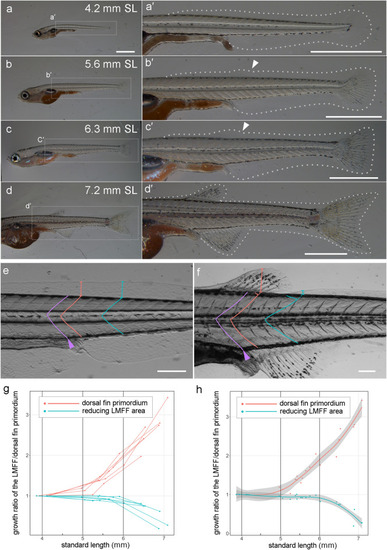
Morphological observation of dorsal fin development and LMFF reduction. ( |
|
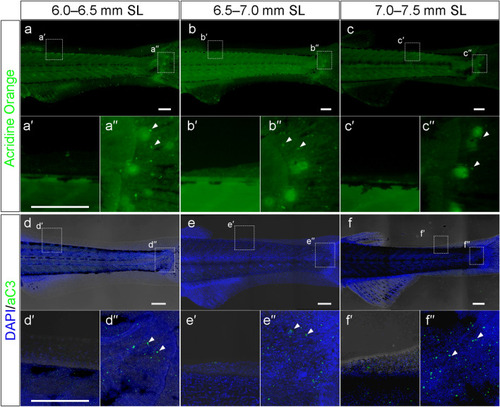
Apoptotic cell death in the reducing LMFF area. ( |
|
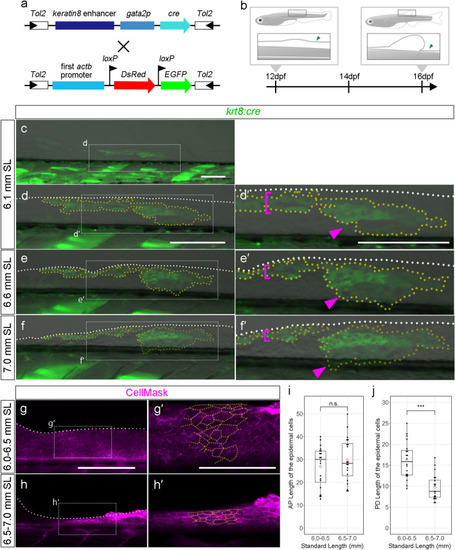
Cell-tracking analysis of the epithelial cells in the reducing LMFF area. ( |
|
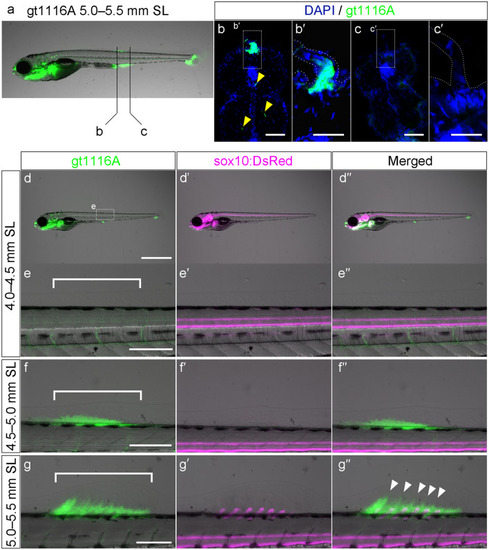
Expression pattern of UAS:EGFP in the |
|
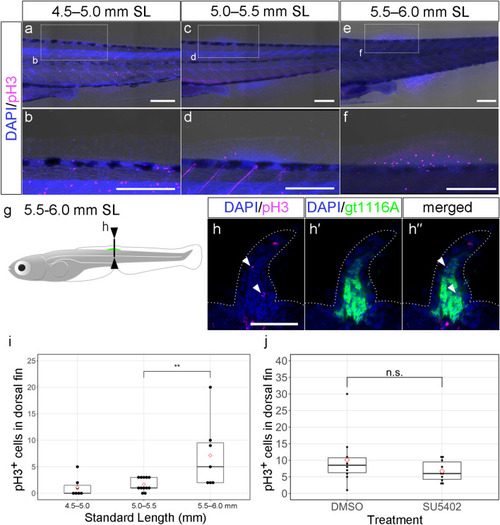
The expression pattern of phospho-histone-H3 in dorsal fin primordium. ( |