- Title
-
Bloom syndrome helicase contributes to germ line development and longevity in zebrafish
- Authors
- Annus, T., Müller, D., Jezsó, B., Ullaga, G., Németh, B., Harami, G.M., Orbán, L., Kovács, M., Varga, M.
- Source
- Full text @ Cell Death Dis.
|
EXPRESSION / LABELING:
|
|
EXPRESSION / LABELING:
PHENOTYPE:
|
|
EXPRESSION / LABELING:
PHENOTYPE:
|
|
|
|
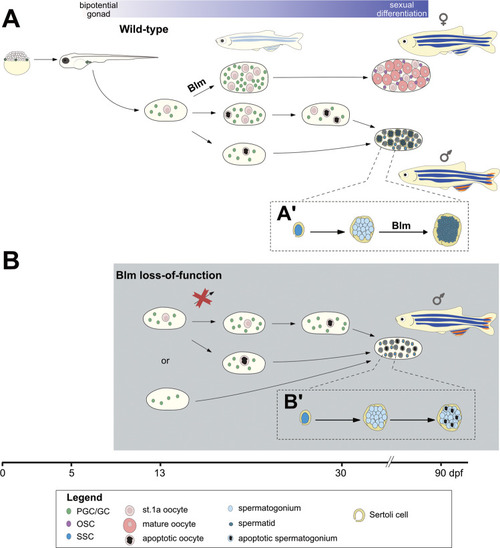
During the early stages of ontogenesis |