- Title
-
Epigenetic dynamics shaping melanophore and iridophore cell fate in zebrafish
- Authors
- Jang, H.S., Chen, Y., Ge, J., Wilkening, A.N., Hou, Y., Lee, H.J., Choi, Y.R., Lowdon, R.F., Xing, X., Li, D., Kaufman, C.K., Johnson, S.L., Wang, T.
- Source
- Full text @ Genome Biol.
|
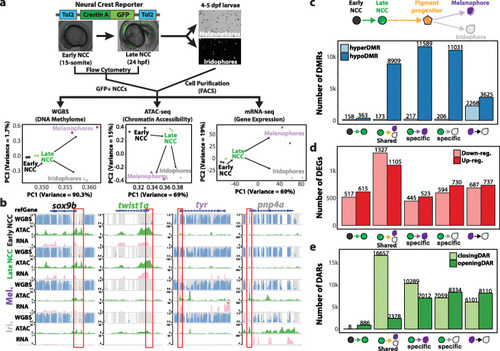
Epigenetic and transcriptomic dynamics of neural crest cell differentiation into pigment cells. EXPRESSION / LABELING:
PHENOTYPE:
|
|
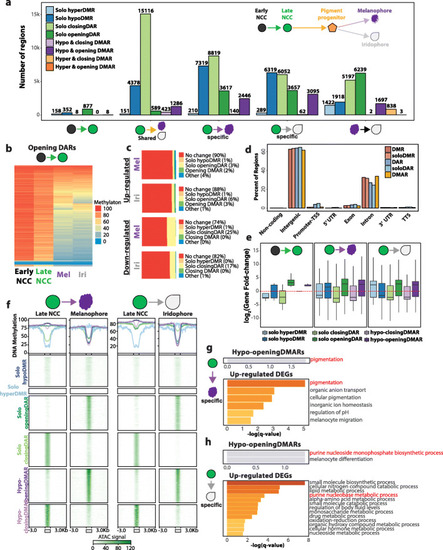
Characterization and annotation of DMARs. a Bar plot illustrating the number of DMARs identified across pigment cell differentiation. No HyperDMARs were detected, except in melanophore vs. iridophore comparison. b Heatmap illustrating the DNA methylation levels of opening DARs identified in early NCC to late NCC transition. c Epigenetic dynamics of DEG promoters in melanophores and iridophores. d Genomic feature distribution of DMRs, DARs, and DMARs. e Expression fold-change of closest DEGs within 50 kb of epigenetically dynamic regions. f Line graphs and heatmaps representing average DNA methylation levels and ATAC peak signals respectively of epigenetically dynamic regions from late NCC to pigment cell-type comparison. g, h Gene ontology enrichment of DEGs within 50 kb of hypo-opening DMARs and upregulated DEGs in melanophore-specific (g) and iridophore-specific (h) comparison PHENOTYPE:
|
|
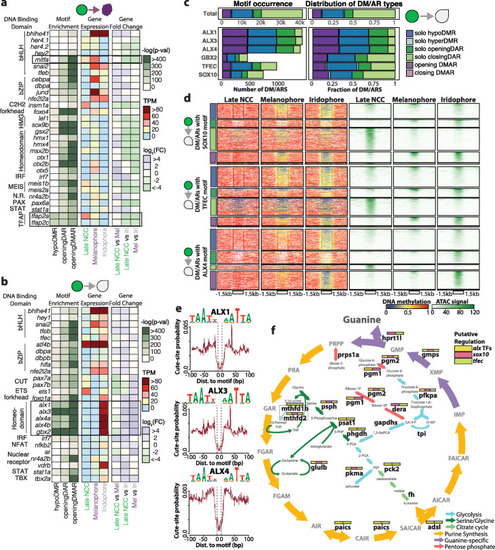
Motif enrichment analysis reveals alx transcription factor family as putative regulator of iridophore development. PHENOTYPE:
|
|
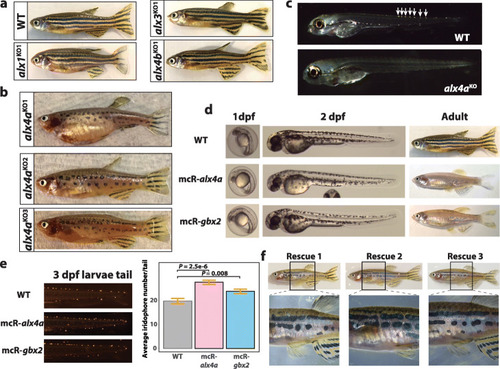
Functional validation of alx4a and gbx2 in iridophore development. |