- Title
-
Locomotion dependent neuron-glia interactions control neurogenesis and regeneration in the adult zebrafish spinal cord
- Authors
- Chang, W., Pedroni, A., Bertuzzi, M., Kizil, C., Simon, A., Ampatzis, K.
- Source
- Full text @ Nat. Commun.
|
|
|
|
|
|
|
|
|
|
|
|
|
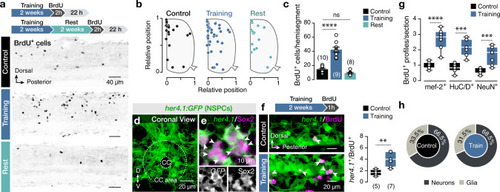
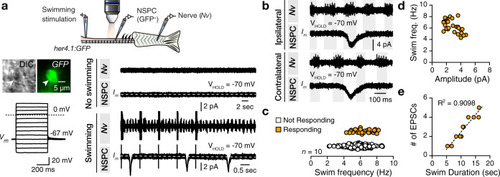
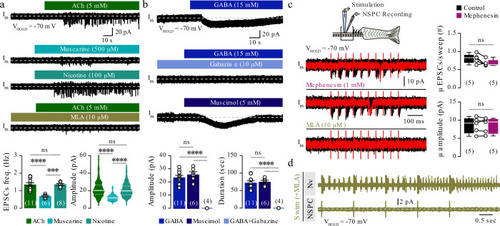
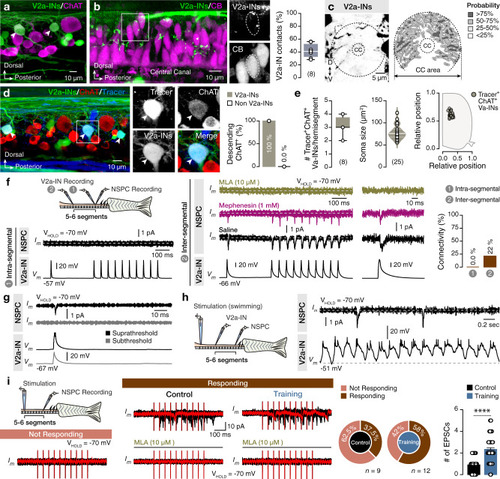
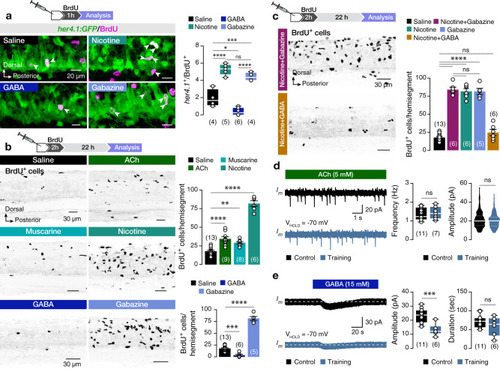
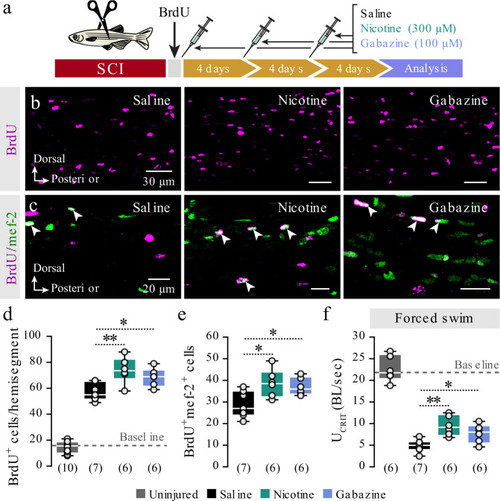
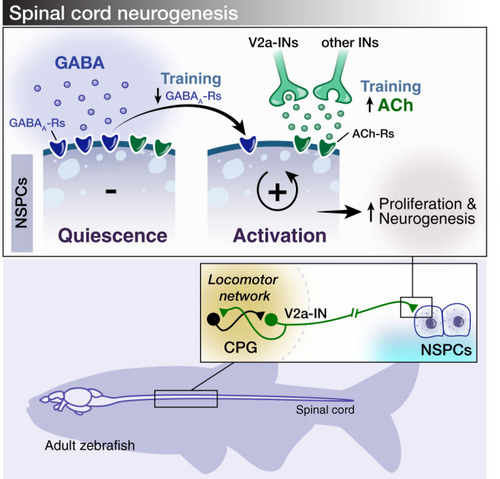
The findings link the locomotor CPG network to adult neurogenesis. Spinal cholinergic interneurons, including the premotor V2a-IN population, increase their cholinergic release to NSPCs during training. ACh acts directly on the NSPCs via nicotinic and muscarinic cholinergic receptors to activate them. Activation of NSPCs leads to the downregulation of GABAA receptors. |