- Title
-
Damage-Induced Calcium Signaling and Reactive Oxygen Species Mediate Macrophage Activation in Zebrafish
- Authors
- Sipka, T., Peroceschi, R., Hassan-Abdi, R., Groß, M., Ellett, F., Begon-Pescia, C., Gonzalez, C., Lutfalla, G., Nguyen-Chi, M.
- Source
- Full text @ Front Immunol
|
|
|
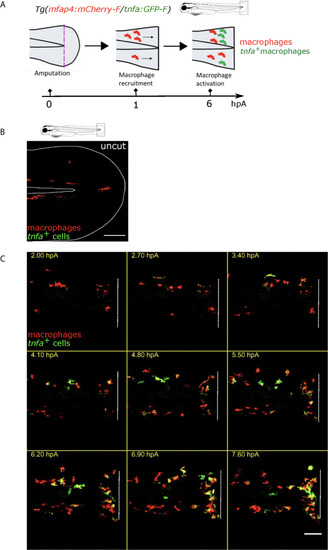
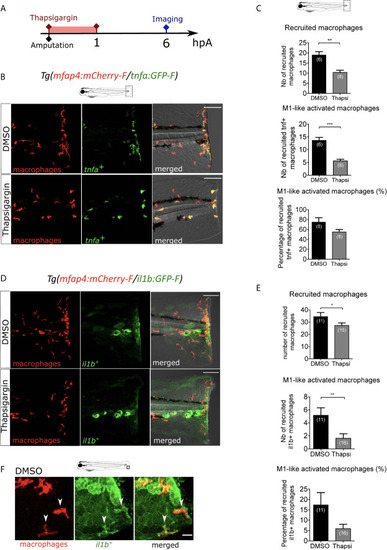
Intracellular Ca2+ signaling mediates macrophage recruitment and activation. |
|
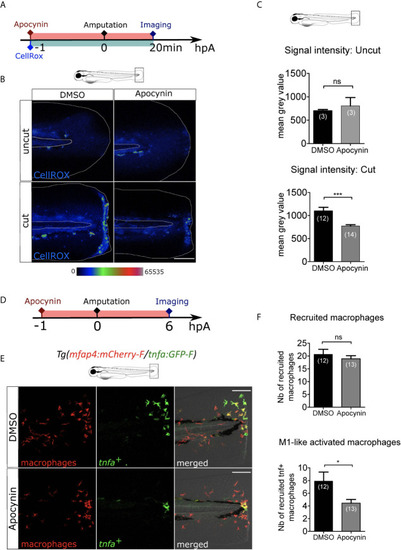
ROS release at the wound mediate macrophage activation but not recruitment. |
|
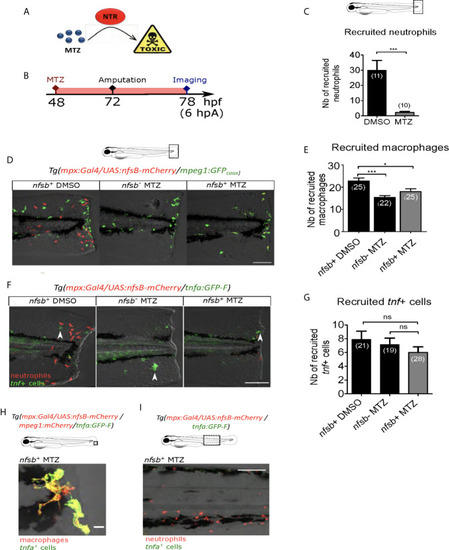
Neutrophil presence at the wound is not necessary for macrophage activation. |
|
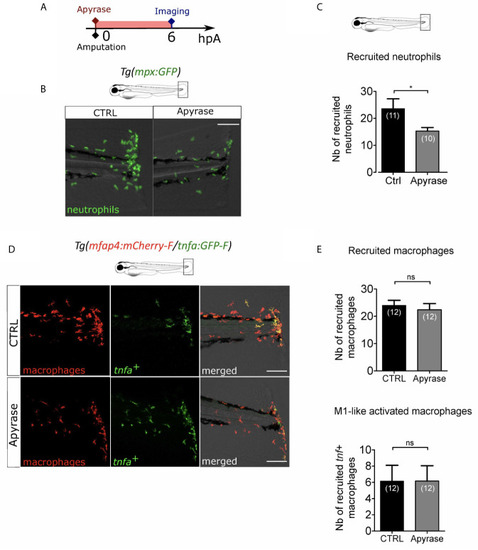
Extracellular ATP is not necessary for macrophage activation. |
|
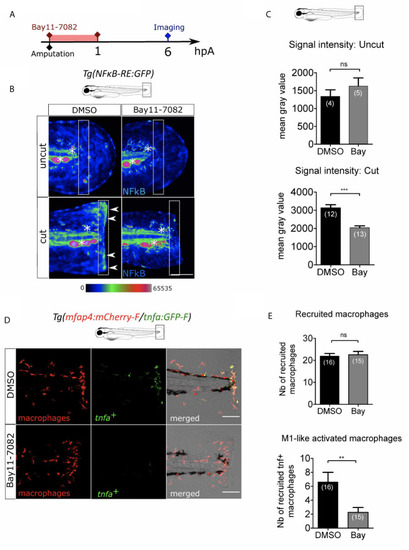
NFκB pathway is enrolled in macrophage activation after wounding. |
|
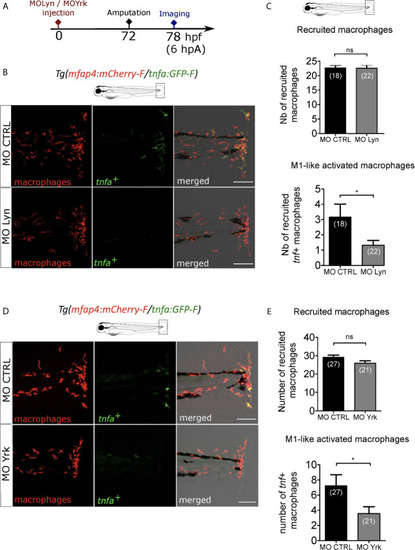
Macrophage-expressed SFKs Lyn and Yrk are enrolled in macrophage activation. |
|
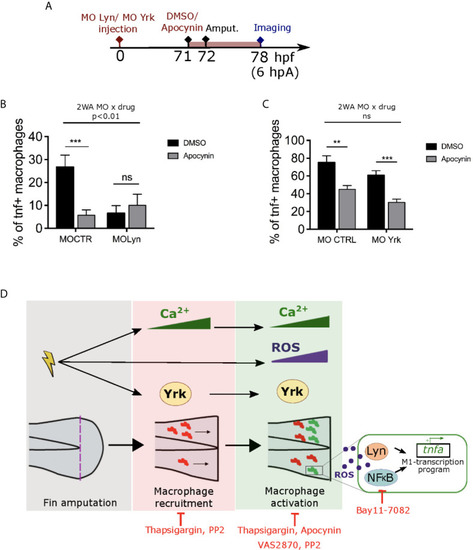
ROS interact with Lyn but not Yrk to mediate macrophage M1-like activation. |