- Title
-
A New Zebrafish Model for Pseudoxanthoma Elasticum
- Authors
- Czimer, D., Porok, K., Csete, D., Gyüre, Z., Lavró, V., Fülöp, K., Chen, Z., Gyergyák, H., Tusnády, G.E., Burgess, S.M., Mócsai, A., Váradi, A., Varga, M.
- Source
- Full text @ Front Cell Dev Biol
|
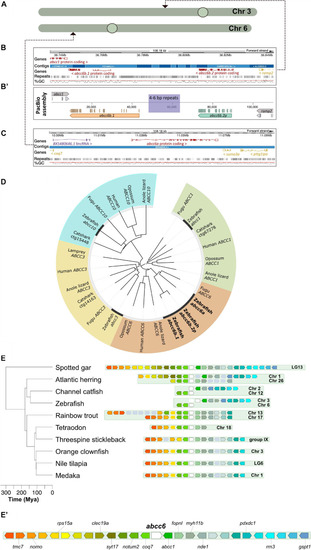
Annotation and phylogenetic relationships of the zebrafish |
|
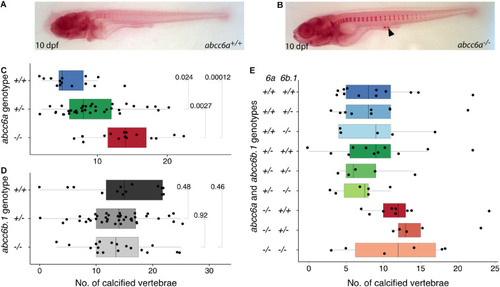
Calcification in single and double mutants of zebrafish PHENOTYPE:
|
|
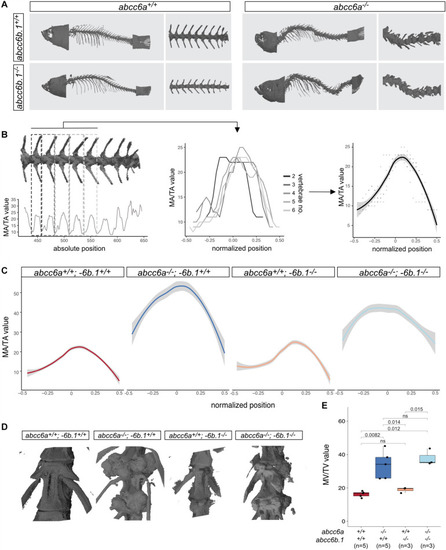
Effects of Abcc6-impairment of skeletal development and life-span. (A) Micro-CT pictures of whole fish and partial vertebral column with indicated genotypes. Abcc6a mutants (regardless of the abcc6b.1 genotype) show easily observable, gross skeletal deformities. (B) A graphical explanation of the method used to calculate normalized MA/TA plots for each genotype. Briefly, MA/TA values calculated for vertebraes 2–6 were averaged to create the normalized plots. (C) Normalized plots of MA/TA values for the indicated genotypes. The results also indicate an increase in the mineralization of the vertebrae of abcc6a–/– fish, while mutations in abcc6b.1 do not seem to affect this process. (D) 3D reconstruction micro-CT images of vertebrae in fish with the indicated genotypes. (E) MV/TV measurements for different genotypes. These results also demonstrate the effect of Abcc6a deficiency of the mineralization of skeletal vertebrae. Abcc6b.1 does not appear to be involved in the same process. (Significance levels calculated with t-test, ns = not significant). PHENOTYPE:
|
|
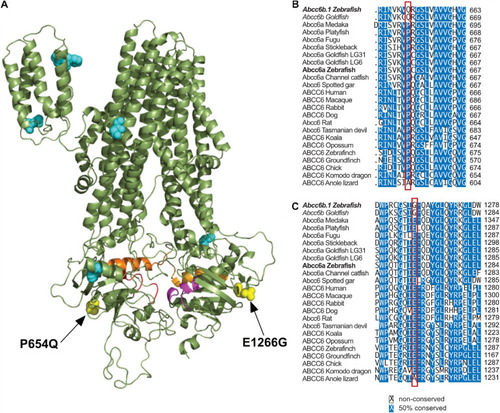
Significant differences in between Abcc6b.1 and canonical ABCC6 sequences. |