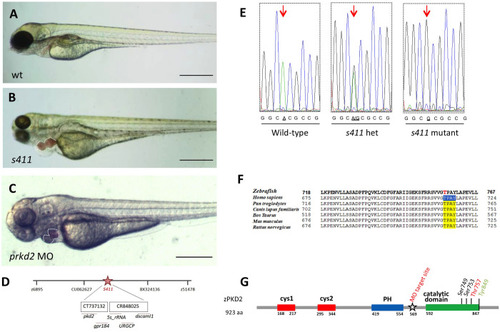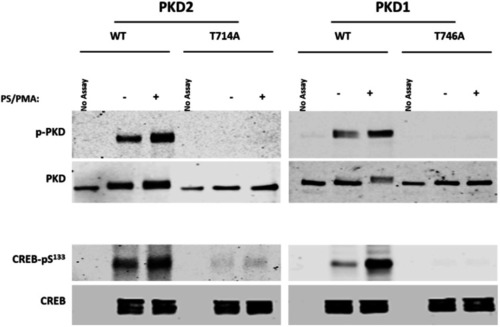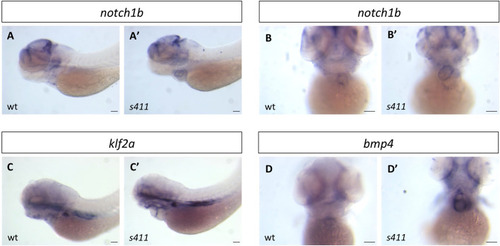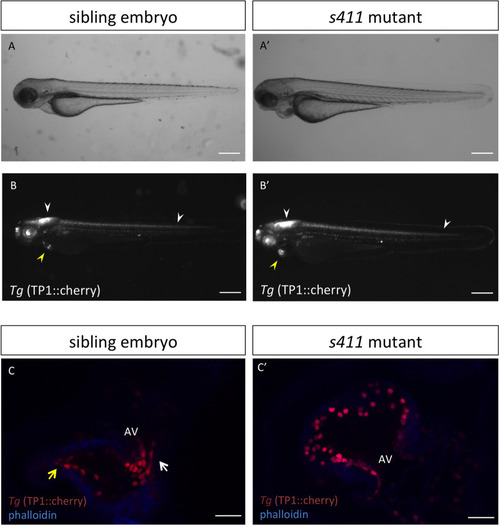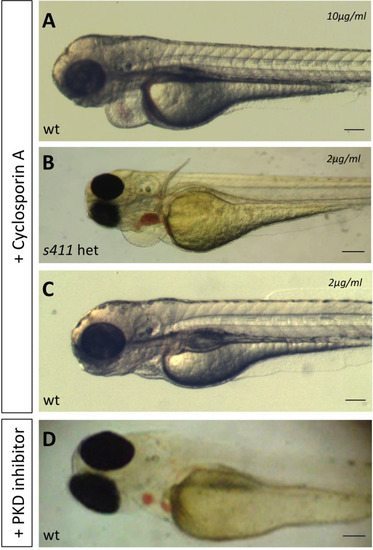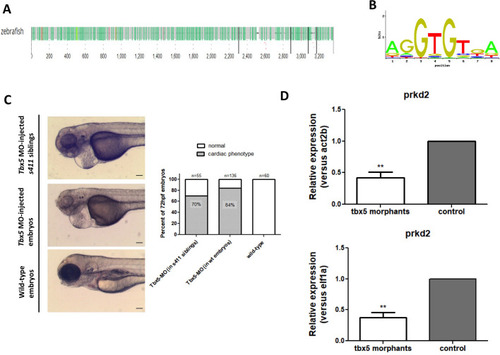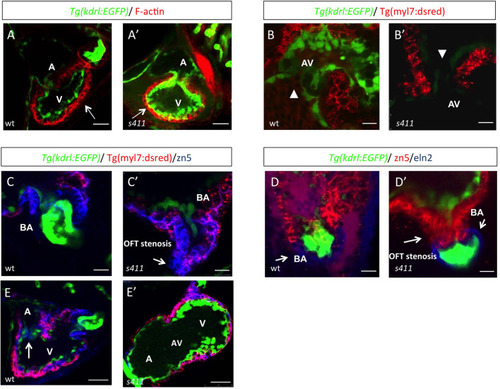
s411 embryos show aortic valve stenosis. (see also Movies 2 and 3). Confocal images of the heart (A,A′,E,E′), the AV canal (B,B′), the outflow track (C,C′) and the bulbus arteriosus (D,D′) at 72 hpf. Tg(kdrl::EGFP) (green) embryos were stained for F-actin (red) (A,A′) or zn5 (pseudo-colored red) and eln2 (pseudo-colored blue) (D,D′). Tg(kdrl::EGFP) (green)/Tg(myl7:DsRed) embryos were stained for zn5 (pseudo-colored blue) (C,C′,E,E′). (A,A′) In s411 embryos, the myocardium appears thinner compared to the wild-type embryos. Arrows indicate the myocardial wall. (B,B′) Endocardial cells residing at AV canal extend in two layers at wild-type embryos whereas the s411 mutants obtain a single-layer of AV endocardial cells. Arrowheads indicate the endocardial cells of AV. (C,C′) The endocardial cells of outflow track are in tight contact in s411 mutant embryos. This structural feature leads to stenosis formation. Arrow indicates the junction between the neighboring endocardial cells. (D,D′) Bulbus arteriosus consists of endocardial cells surrounded by a layer of smooth muscle cells. s411 mutants have a blocked bulbus arteriosus. Arrows show the elastin-positive cells and the stenosis of mutant embryos. (E,E′) In s411 embryos, the endocardial monolayer of AV cells does not express zn5 compared to the two-layer zn5+ AV endocardial cells in wild-type embryos, which indicates the malformation of atrioventricular cardiac valve during development. A, atrium; V, ventricle; AV, atrioventricular, BA, bulbus arteriosus. n=10 in each of three independent experiments. Scale bars: 50 μm for A,A′, E,E′ and 20 μm for B–D′.
|