- Title
-
Vasohibin-1 selectively regulates secondary sprouting and lymphangiogenesis in the zebrafish trunk
- Authors
- Bastos de Oliveira, M., Meier, K., Jung, S., Bartels-Klein, E., Coxam, B., Geudens, I., Szymborska, A., Skoczylas, R., Fechner, I., Koltowska, K., Gerhardt, H.
- Source
- Full text @ Development
|
|
|
|
|
|
|
|
|
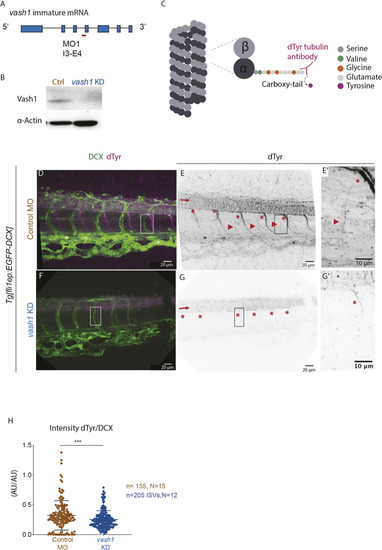
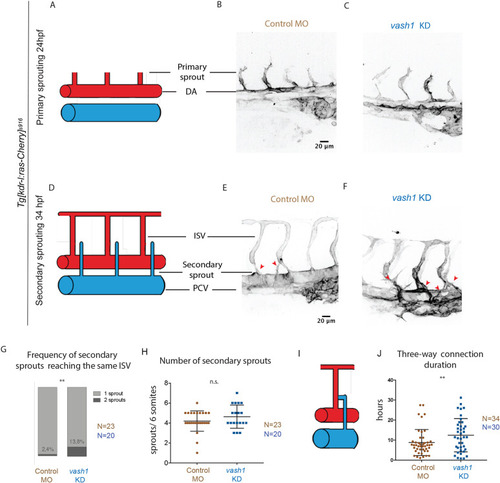
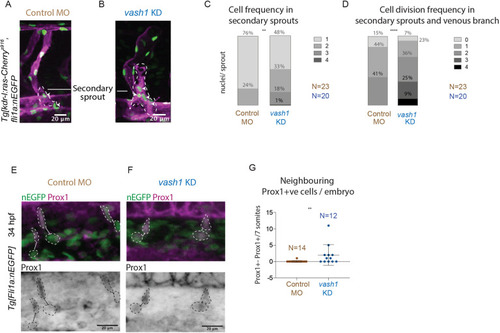
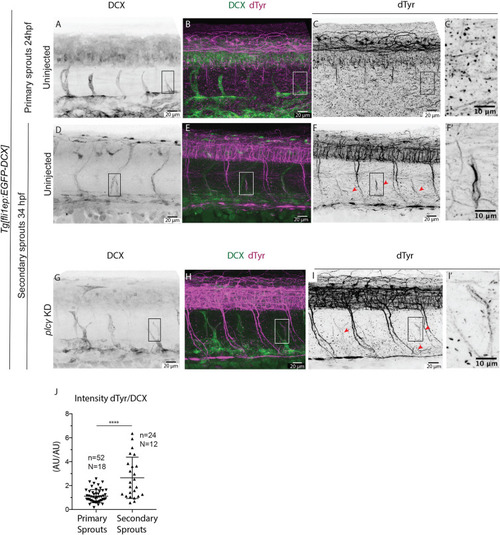
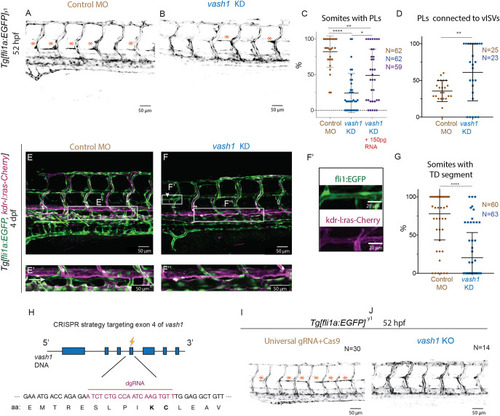
Microtubules of secondary sprouts are selectively detyrosinated. (A-I′) Immunostainings using antibody detecting the glutamate amino acid of detyrosinated microtubules (dTyr) during primary (A-C) and secondary (D-I) sprouting in uninjected (A-F) and plcγ KD (G-I) Tg[fli1ep:EGFP-DCX] embryos, labelling all endothelial microtubules (DCX). C′,F′ and I′ are magnifications from boxed region in C,F,I, respectively. plcγ KD embryos (G-I′) show reduced primary sprouting, facilitating the visualization and quantification of dTyr signal specifically in secondary sprouts. Arrowheads indicate secondary sprouts. (J) Quantification of the dTyr/DCX signal intensities in primary sprouts of control MO-injected embryos, and secondary sprouts from plcγ MO-injected embryos. n=52 control primary sprouts from 18 embryos, n=24 plcγ KD secondary sprouts from 12 embryos, from one experiment. ****<0.00001 (Mann–Whitney test). Pictures are representative of 3 replicated experiments. |
|
|
|
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |