- Title
-
Loss of CBY1 results in a ciliopathy characterized by features of Joubert syndrome
- Authors
- Epting, D., Senaratne, L.D.S., Ott, E., Holmgren, A., Sumathipala, D., Larsen, S.M., Wallmeier, J., Bracht, D., Frikstad, K.M., Crowley, S., Sikiric, A., Barøy, T., Käsmann-Kellner, B., Decker, E., Decker, C., Bachmann, N., Patzke, S., Phelps, I.G., Katsanis, N., Giles, R., Schmidts, M., Zucknick, M., Lienkamp, S.S., Omran, H., Davis, E.E., Doherty, D., Strømme, P., Frengen, E., Bergmann, C., Misceo, D.
- Source
- Full text @ Hum. Mutat.

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
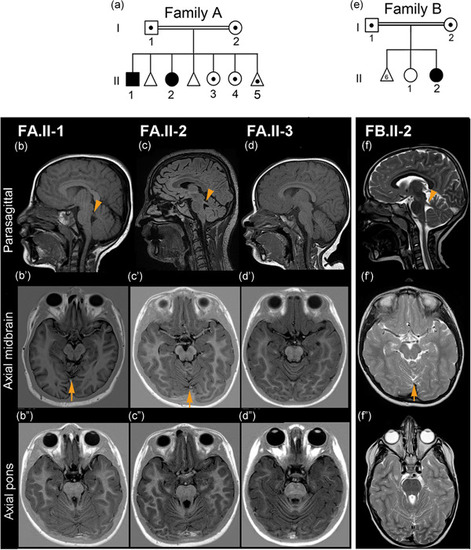
Pedigree of Families A and B and cerebral MRI findings. (a) Pedigree of Family A showing the two affected siblings (black symbols) and carriers (symbols with the dot) of the |
|
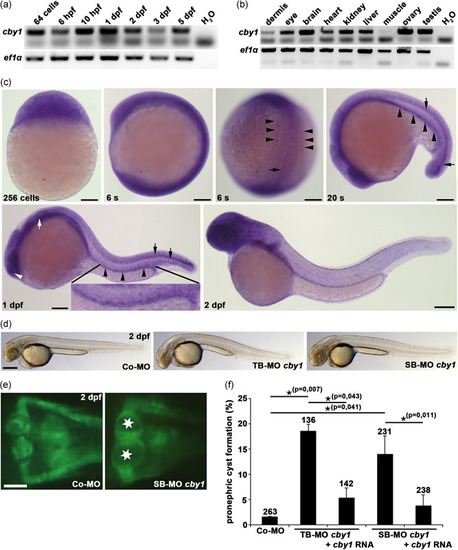
Zebrafish studies showing temporal and spatial expression of |
|
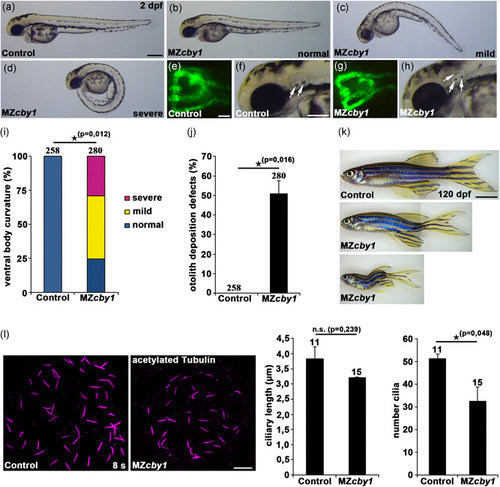
Zebrafish PHENOTYPE:
|
|
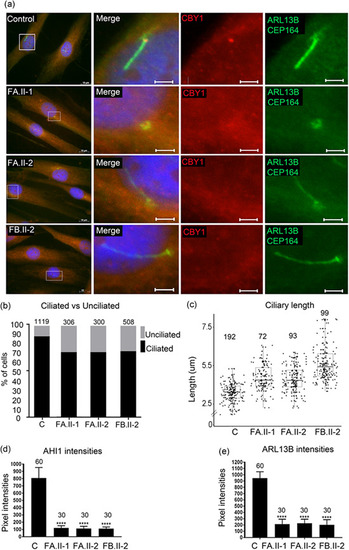
Reduced fraction of ciliated cells and increased primary cilia length in fibroblasts from patients. (a) Fibroblasts from two controls (images from one control shown) and from the individuals FA.II‐1, FA.II‐2, FB.II‐2 were fixed after 72 h of serum starvation and stained for ARL13B and CEP164 (Alexa488, green), CBY1 (Cy3, red), and nuclei (Hoechst, blue). The CBY1 signal was detected at the transition zone in the control, but not in cells from FA.II‐1, FA.II‐2, FB.II‐2. Scale bars are 10 µm in the left panel and 2 µm in the magnified pictures. (b) Fibroblasts from two controls and from the affected individuals FA.II‐1, FA.II‐2, FB.II‐2 were fixed after 72h serum starvation and stained for acetylated tubulin, CEP164, and nuclei (Hoechst). The numbers of ciliated fibroblasts were significantly reduced in FA.II‐1, FA.II‐2, and FB.II‐2 ( |