- Title
-
Controlling horizontal cell-mediated lateral inhibition in transgenic zebrafish retina with chemogenetic tools
- Authors
- Beckwith-Cohen, B., Holzhausen, L.C., Nawy, S., Kramer, R.H.
- Source
- Full text @ eNeuro
|
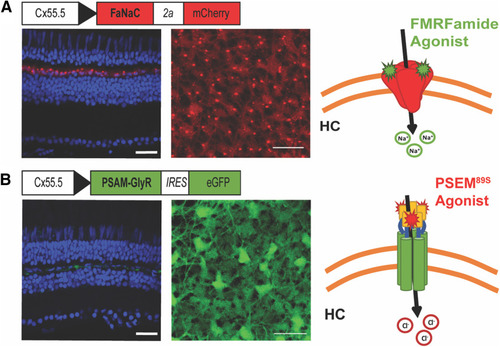
Strategy for chemogenetic manipulation of HC membrane potential. |
|
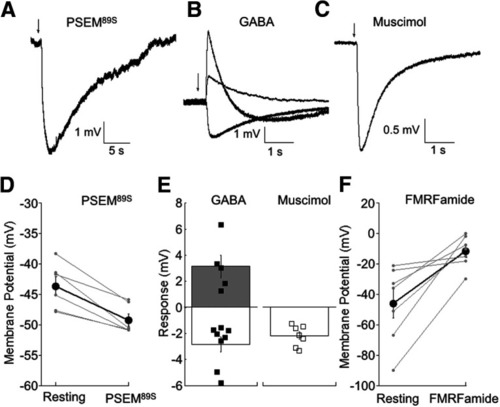
Monitoring changes in membrane potential elicited by FMRFamide and PSEM89S. |
|
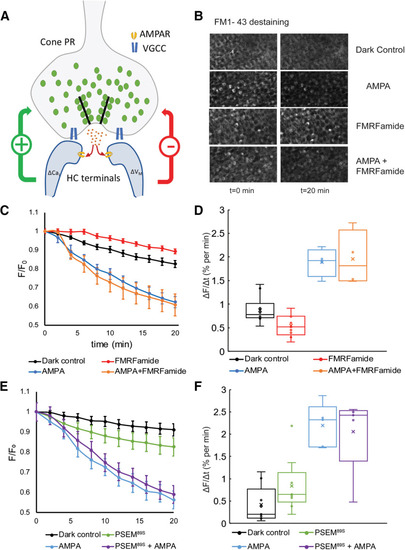
Chemogenetic activation of HCs alters vesicular release of FM1-43 from cone terminals. |
|
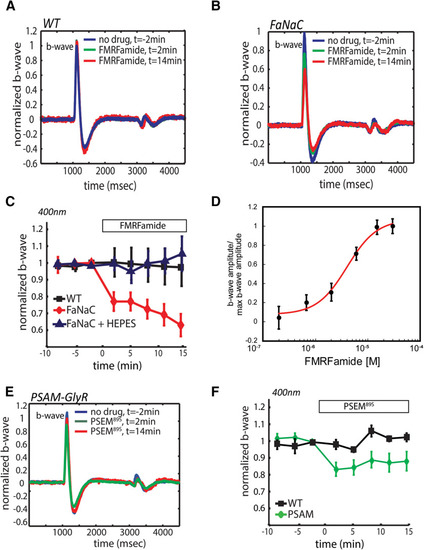
Chemogenetic manipulation of HCs modulates the bipolar cell light response. Application of FMRFamide had no effect on the ERG of WT zebrafish ( |
|
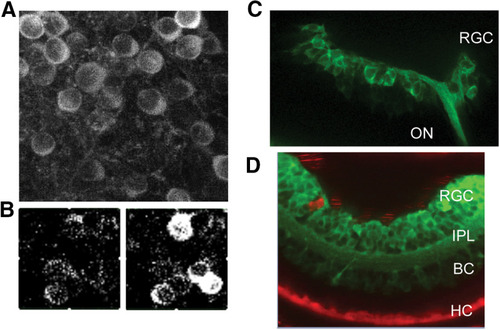
Expression of GCaMP6f in chemogenetic zebrafish lines. |
|
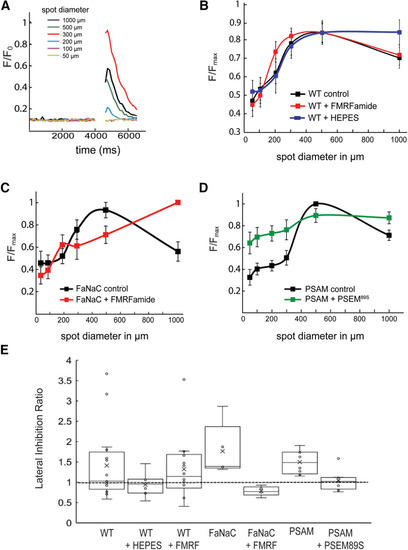
Chemogenetic manipulation of HCs alters lateral inhibition in downstream RGCs. |