- Title
-
Autophagic flux inhibition enhances cytotoxicity of the receptor tyrosine kinase inhibitor ponatinib
- Authors
- Corallo, D., Pastorino, F., Pantile, M., Mariotto, E., Caicci, F., Viola, G., Ponzoni, M., Tonini, G.P., Aveic, S.
- Source
- Full text @ J. Exp. Clin. Cancer Res.
|
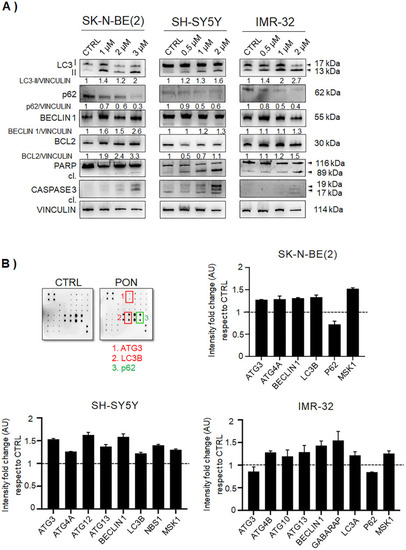
PON induces autophagy in neuroblastoma cells. |
|
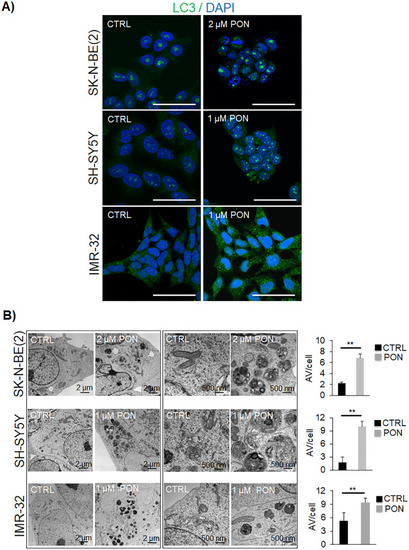
PON promotes autophagy vesicle accumulation in human neuroblastoma cells |
|
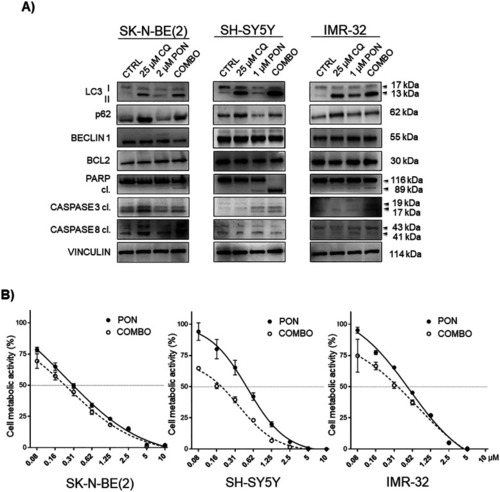
CQ interrupts PON-induced autophagy and sensitizes neuroblastoma cells to PON-dependent cytotoxicity. |
|
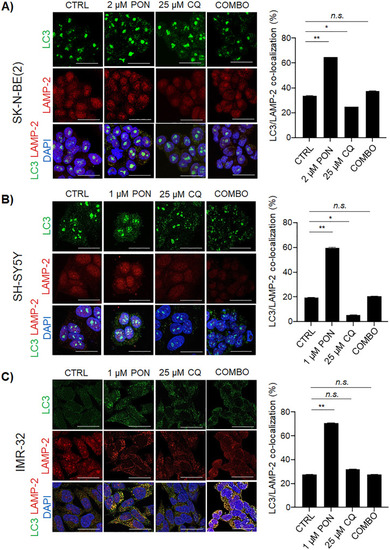
Combo therapy interferes with autophagy flux in human neuroblastoma cells. |
|
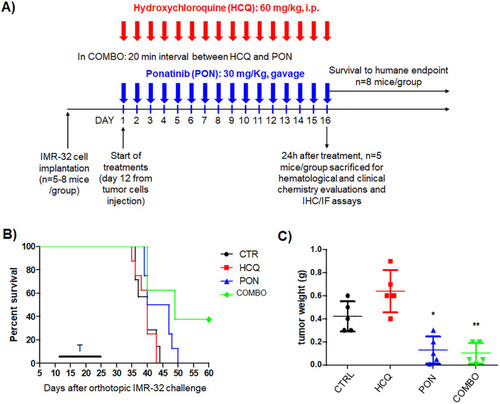
CQ potentiates PON-induced cytotoxicity in the neuroblastoma mice model. |
|
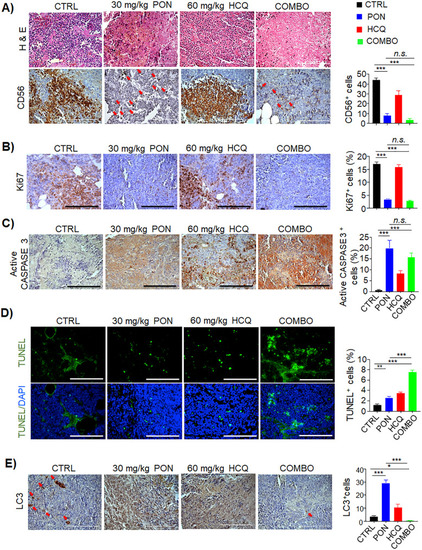
Histopathological examination of post-therapy neuroblastoma tumors confirms the efficacy of combination treatment in vivo. |