- Title
-
Enteric glia as a source of neural progenitors in adult zebrafish
- Authors
- McCallum, S., Obata, Y., Fourli, E., Boeing, S., Peddie, C.J., Xu, Q., Horswell, S., Kelsh, R., Collinson, L., Wilkinson, D., Pin, C., Pachnis, V., Heanue, T.A.
- Source
- Full text @ Elife
|
( |
|
( |
|
( |
|
( |
|
( |
|
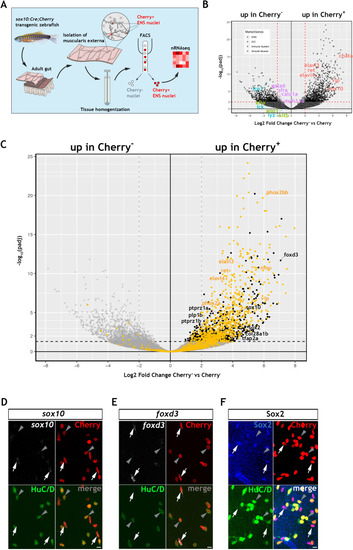
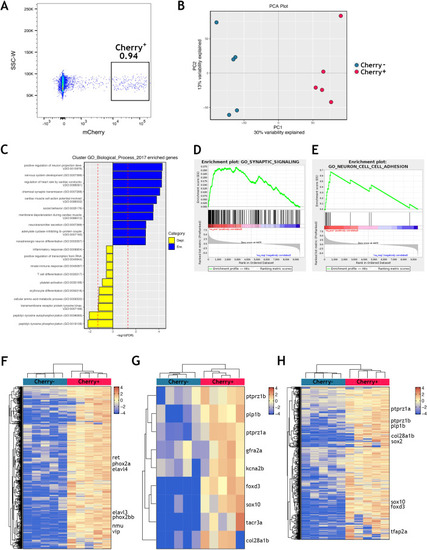
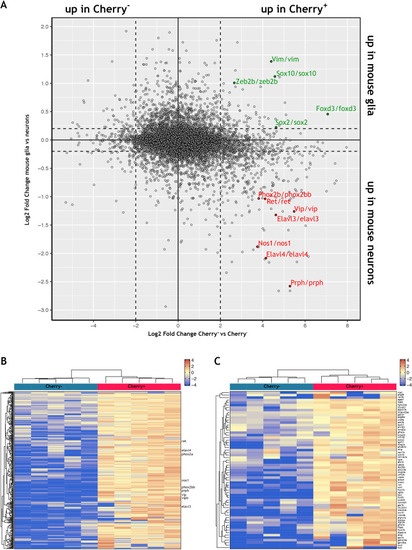
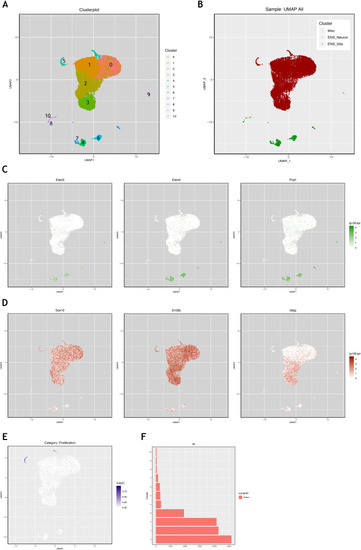
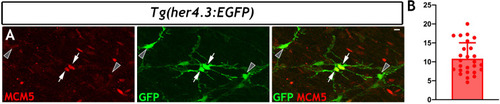
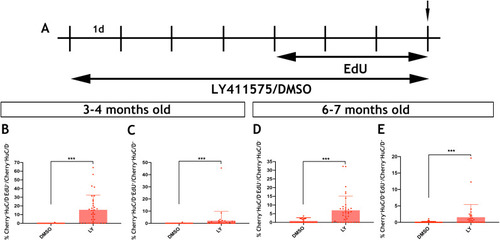
Using the mouse single cell transcriptomic data from |
|
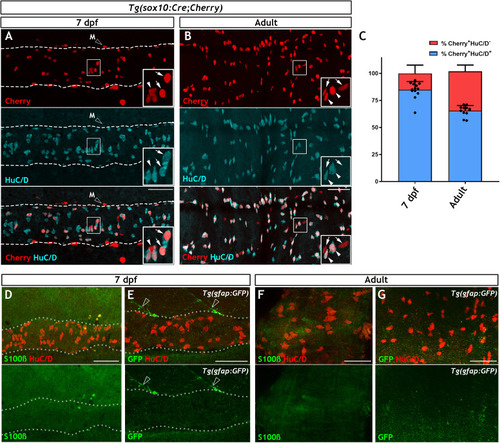
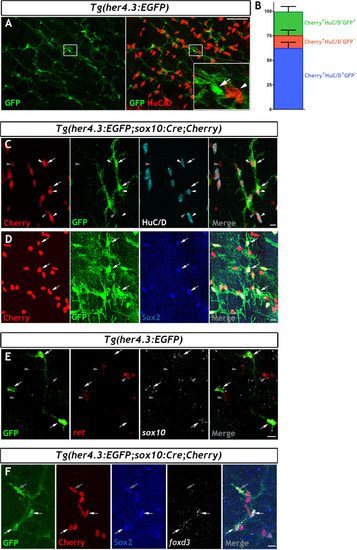
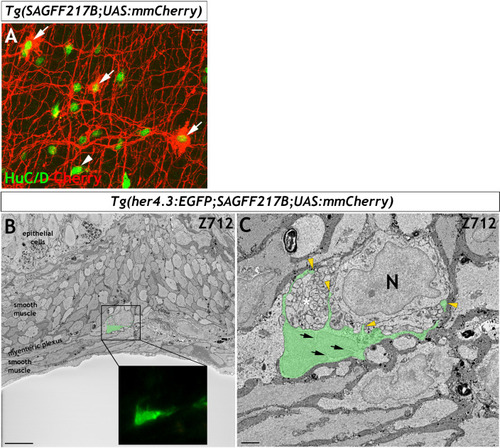
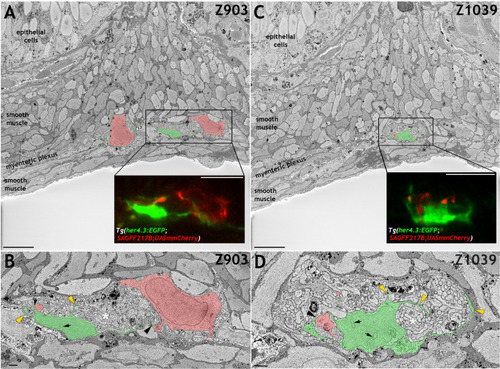
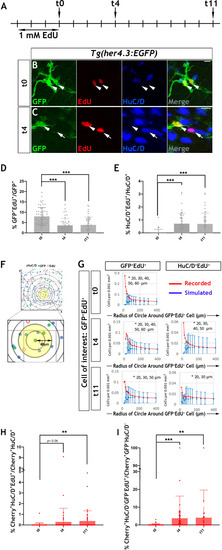
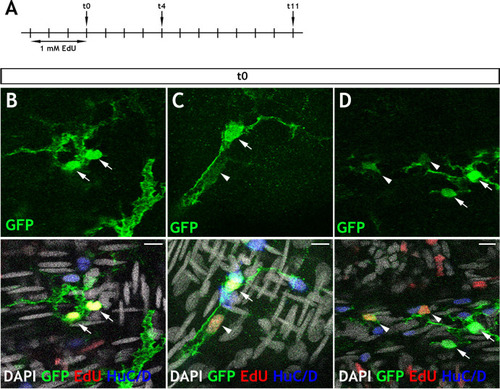
Immunohistochemistry of adult guts from of |
|
Immunohistochemistry of adult guts from of |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
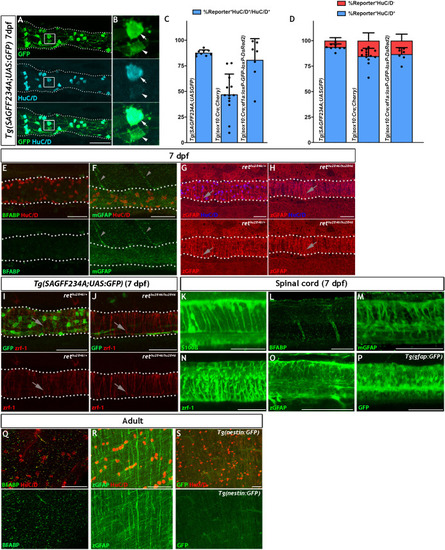
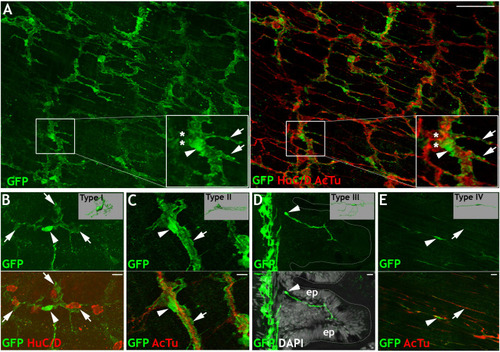
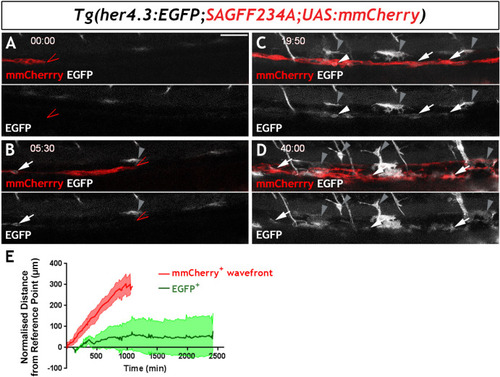
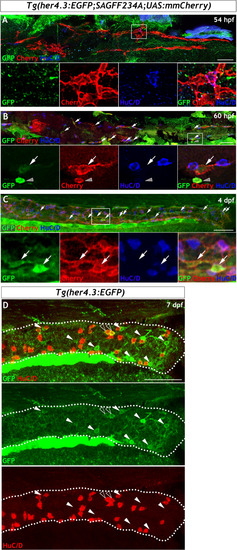
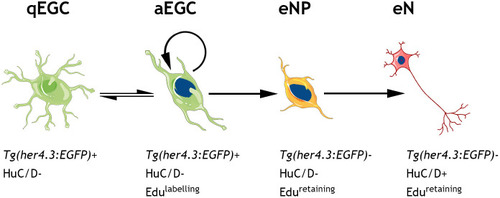
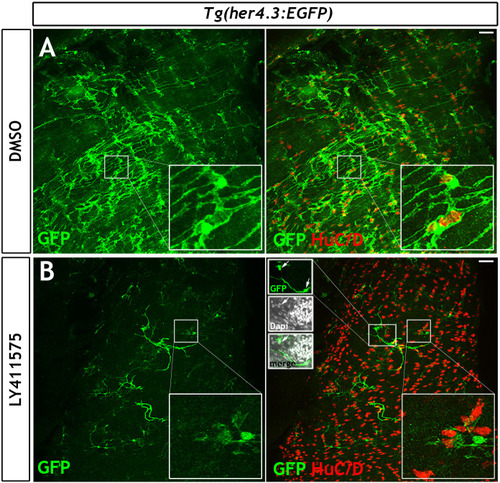
Given the similarities between |
|
( |
|
( |