- Title
-
Deletion of cftr Leads to an Excessive Neutrophilic Response and Defective Tissue Repair in a Zebrafish Model of Sterile Inflammation
- Authors
- Bernut, A., Loynes, C.A., Floto, R.A., Renshaw, S.A.
- Source
- Full text @ Front Immunol
|
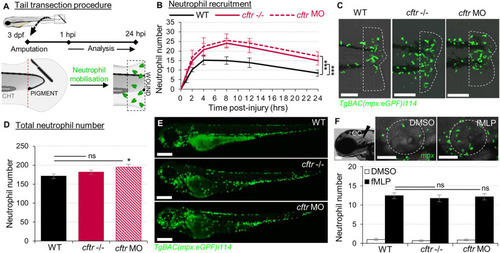
Sterile injury-induced neutrophilic inflammatory responses are exacerbated in the absence of CFTR. PHENOTYPE:
|
|
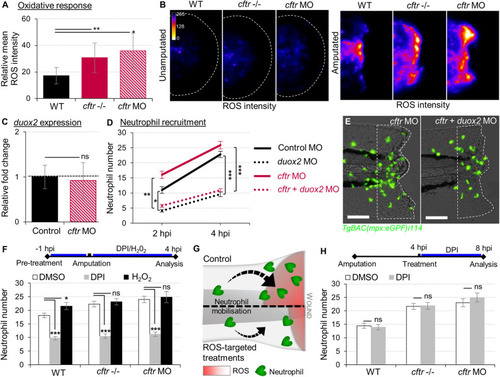
Exuberant wound-induced oxidative responses drive the overactive neutrophilic response in CF larvae. EXPRESSION / LABELING:
PHENOTYPE:
|
|
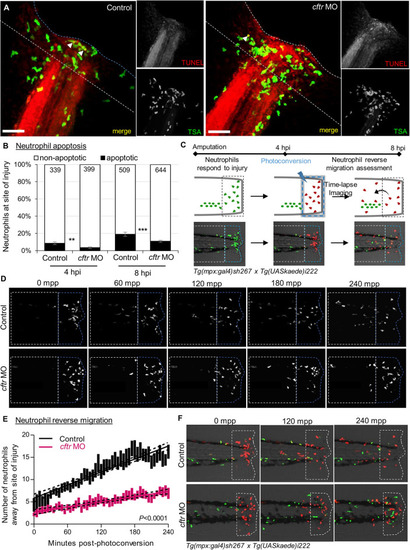
CFTR deficiency delays inflammation resolution PHENOTYPE:
|
|
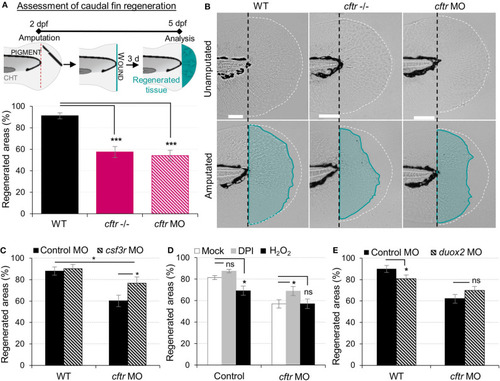
Neutrophilic response in CF hampers tissue repair PHENOTYPE:
|
|
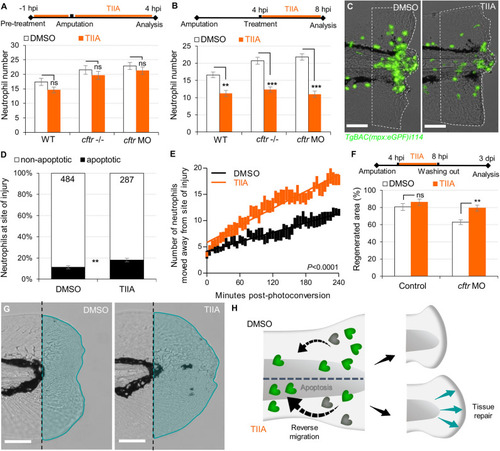
TIIA-driven neutrophil apoptosis and reverse migration accelerate inflammation resolution in CF. PHENOTYPE:
|
|
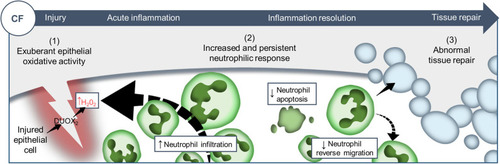
Proposed model showing CF-related inflammation immuno-pathogenesis. The spatiotemporal events associated with CFTR ablation reveal a mechanism whereby CFTR participates in the adjustment of innate immunity, conditioning key regulatory mediators involved in the regulation of inflammation and tissue repair. A sterile lesion is characterized by early release of epithelial H2O2 through DUOX2, leading to neutrophil recruitment towards wounds; coordination of neutrophil apoptosis and reverse migration promoting efficient inflammation resolution, and allowing to restore tissue homeostasis and initiate tissue repair. In contrast, in CF zebrafish sterile injury leads to extensive inflammation, typified by increased then sustained accumulation of neutrophils at wounds: (1) excessive epithelial ROS release drives increased neutrophil recruitment towards wounds; (2) reduction of neutrophil apoptosis and impaired retrograde migration of neutrophils resulting in delayed resolution of inflammation. (3) Therefore, the increased number of neutrophils that mobilize in an uncontrolled manner at wound sites causes persistent inflammation, severe tissue damage, and abnormal tissue repair. |