- Title
-
Conserved and Divergent Features of Adult Neurogenesis in Zebrafish
- Authors
- Labusch, M., Mancini, L., Morizet, D., Bally-Cuif, L.
- Source
- Full text @ Front Cell Dev Biol
|
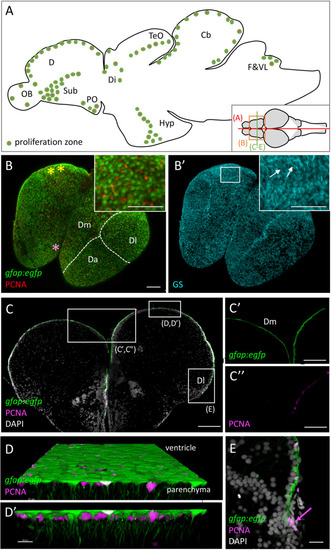
Progenitor cells in the zebrafish adult brain at 3 months-post-fertilization (mpf). |
|
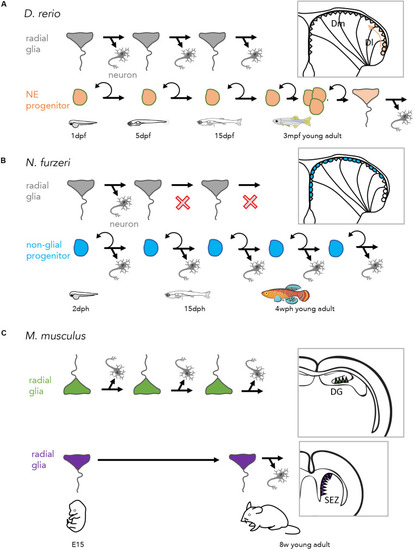
Lineages at the origin of adult neurogenic progenitors in the vertebrate pallium. |
|
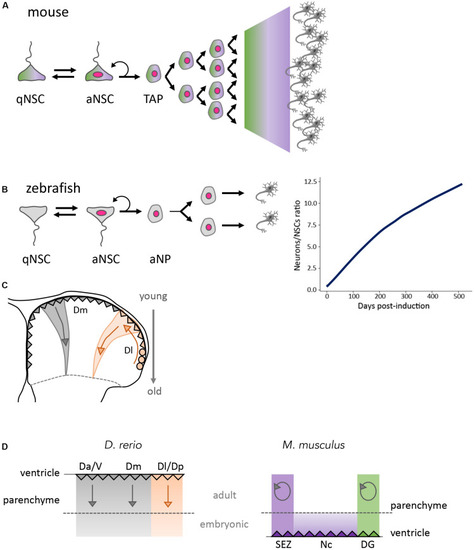
Global outputs of adult neurogenesis in zebrafish and mouse. |
|
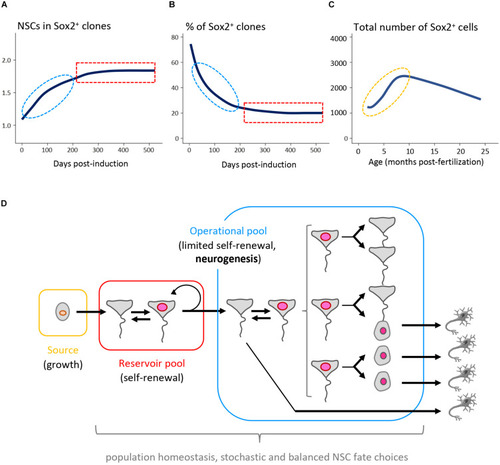
Long-term NSC and neurogenesis dynamic in the adult zebrafish pallium. |
|
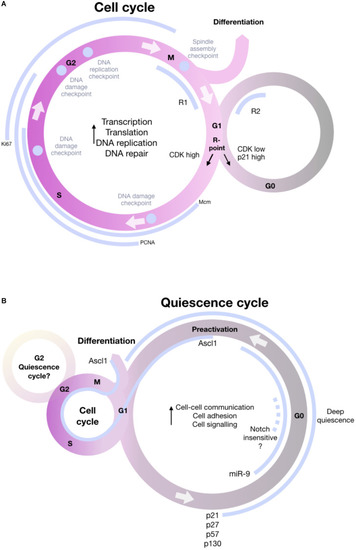
Schematic of the cell cycle including the most important information about the decision to enter quiescence, remain in cycle or differentiate. |
|
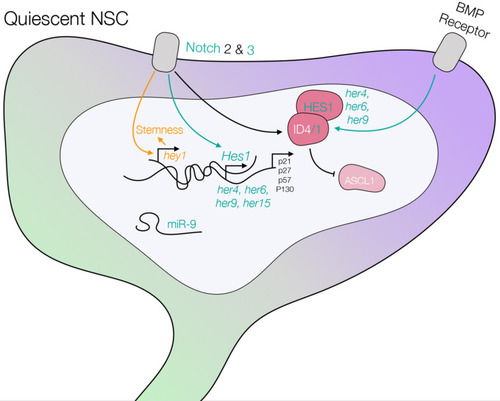
Schematic of a quiescent NSC including the pathways controlling quiescence, which are summarized in this review. The scheme highlights knowledge generated in mouse, and confirmed pathways in zebrafish are illustrated in green. Knowledge generated in zebrafish and later extended to mouse is shown in green as well. Knowledge generated in zebrafish and still to be confirmed in mouse is depicted in yellow. Differences (Notch2 is not expressed in qNSCs in zebrafish), or data that need consolidation in zebrafish (Ascl1 expression and its regulation by ID and Hes1, BMP receptor), are shown in black. See text and |
|
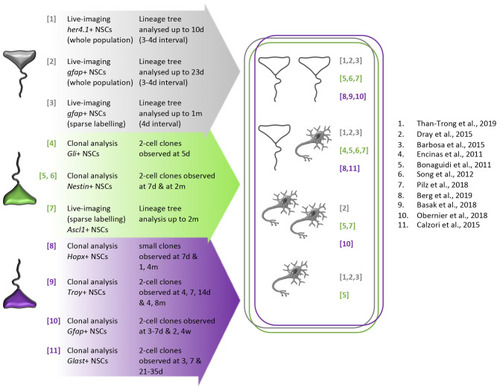
Schematic summary of division modes directly observed in adult mouse telencephalic neurogenic niches and in the zebrafish adult pallium |
|
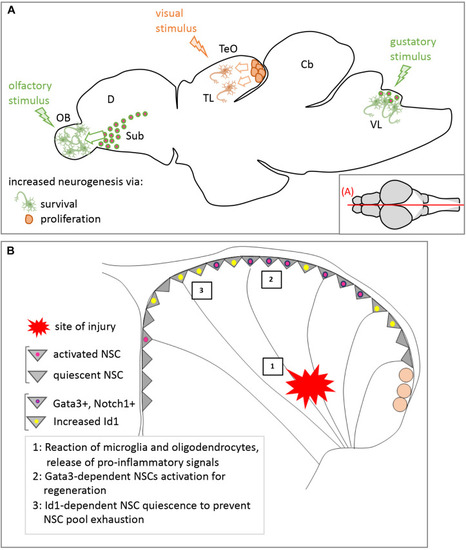
Modulation of adult neurogenesis by external stimuli in zebrafish. |