- Title
-
Validating the Paradigm That Biomechanical Forces Regulate Embryonic Cardiovascular Morphogenesis and Are Fundamental in the Etiology of Congenital Heart Disease
- Authors
- Keller, B.B., Kowalski, W.J., Tinney, J.P., Tobita, K., Hu, N.
- Source
- Full text @ J Cardiovasc Dev Dis
|
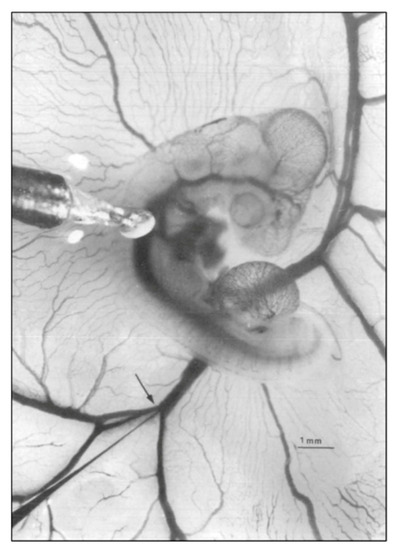
Representative photo of a stage 21 chick embryo for hemodynamic measurements. A 1-mm diameter piezoelectric crystal is positioned over the dorsal aorta at a 45 °angle and a 5 mm diameter tip glass micropipette is positioned in the left vitelline vein. Scale bar = 1 mm. This image was reproduced with permission [ |
|
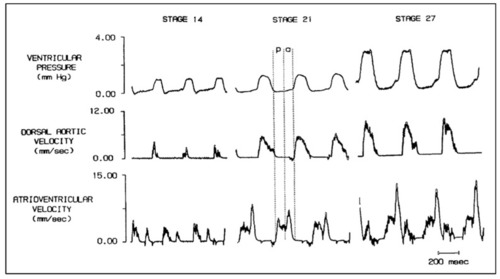
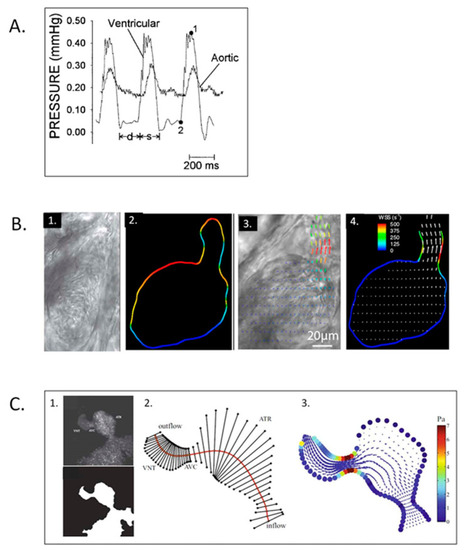
Simultaneous ventricular pressure, dorsal aortic velocity and atrioventricular velocity in stage 14, 21, and 27 chick embryos. Diastole is partitioned into the passive filling phase ( |
|
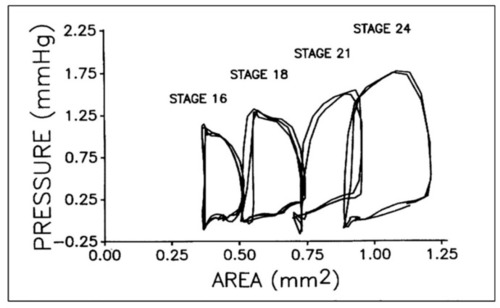
Representative pressure–area loops for stage 16 to 24 chick embryos. Two or three cardiac cycles are included for each stage as synchronized, raw data. This was reproduced with permission [ |
|
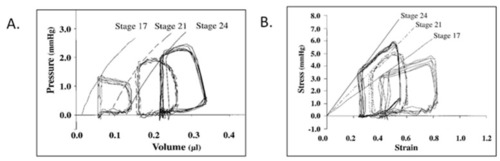
Representative pressure–volume ( |
|
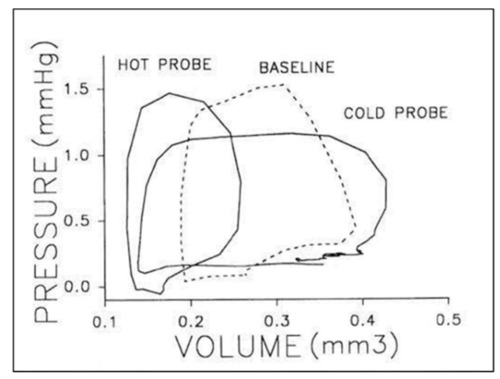
Representative pressure–volume loops for a stage 21 chick embryo at baseline (dashed line), intrinsic heart rate then in response thermal probe application to increased (hot) or decreased (cold) heart rate. Two or three cardiac cycles are included for each stage as synchronized, raw data. This was reproduced with permission [ |
|
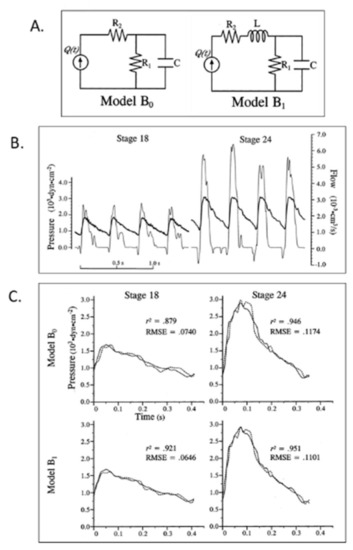
Lumped parameter Windkessel estimation of embryonic chick arterial impedance. ( |
|
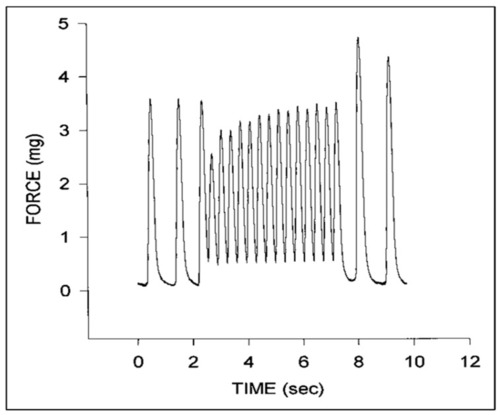
Representative force tracings for a stage 24 chick embryo myocardial specimen. Note the initial increase in diastolic force and decrease in systolic force at higher pacing rates that gradually increases with time and the increase in peak systolic force upon return to the intrinsic pacing rate. This was adapted with permission [ |
|
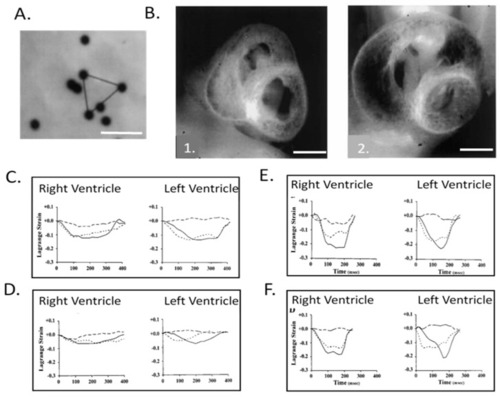
Right and left ventricular epicardial strains in normal and left heart hypoplasia chick embryos. ( |
|
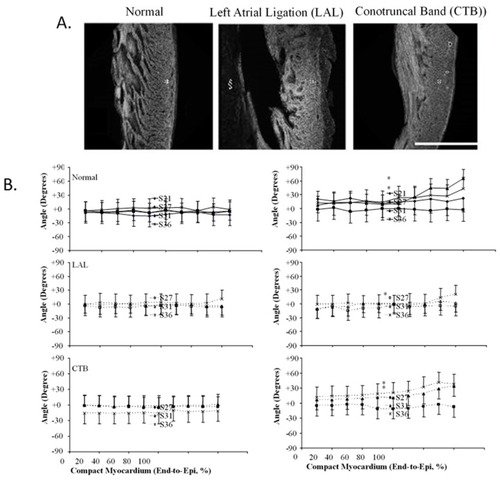
Three-dimensional myofiber architecture of the embryonic LV during normal development and altered mechanical loads. ( |
|
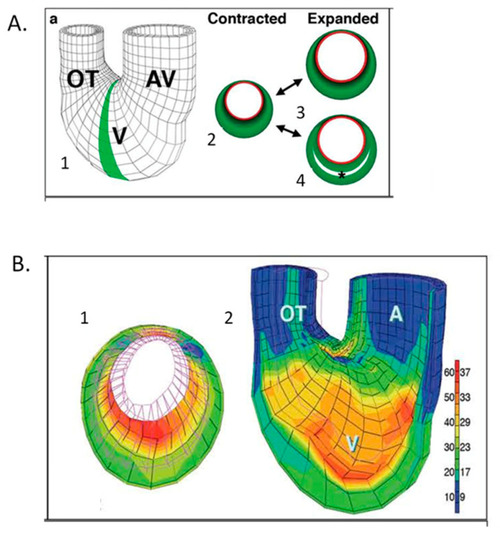
Computational modeling of embryonic heart wall strains. ( |
|
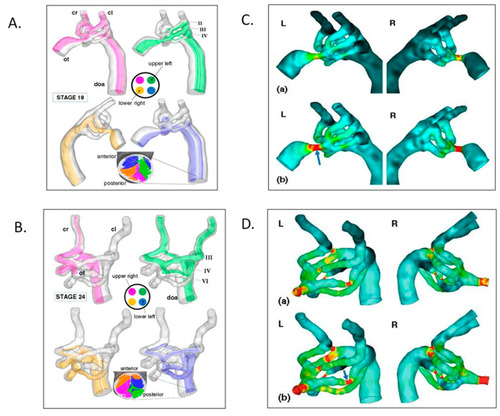
Aortic arch morphogenesis and flow modeling. ( |
|
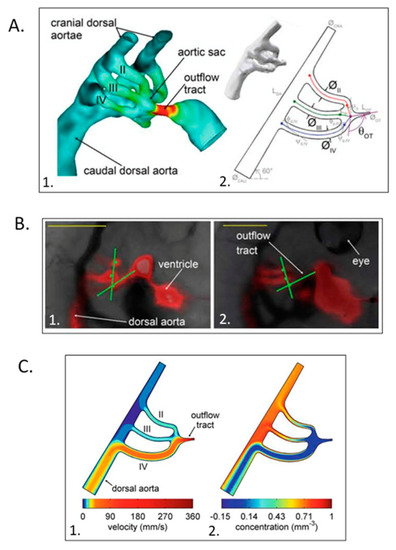
Computational hemodynamic optimization predicts embryonic chick aortic arch selection. ( |
|
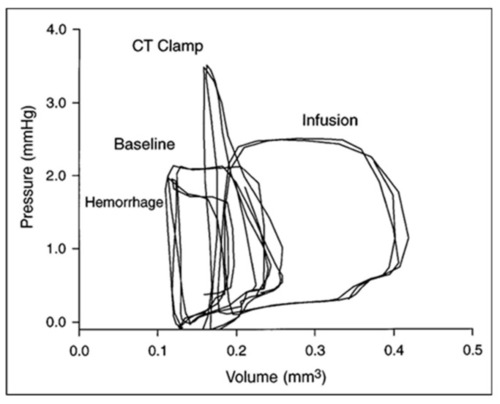
Ventricular–vascular uncoupling during acute conotruncal (CT) occlusion. Representative pressure–volume loops at baseline, during buffer infusion, and after CT clamp in a stage 21 chick embryo. Note the increased end-systolic pressure despite reduced stroke volume during CT clamp consistent with contractile reserve and only a modest increase in end-systolic pressure during infusion consistent with a curvilinear end-systolic PV relation. This was adapted with permission [ |
|
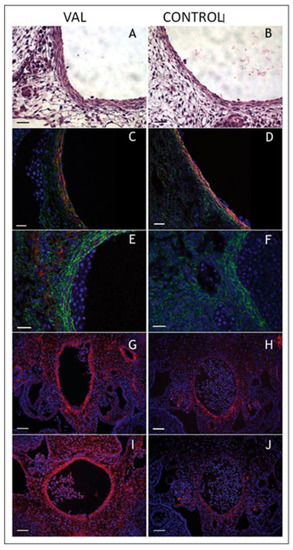
Increased arterial load via unilateral vitelline artery ligation (VAL) alters aortic structural properties. Representative images of VAL ( |
|
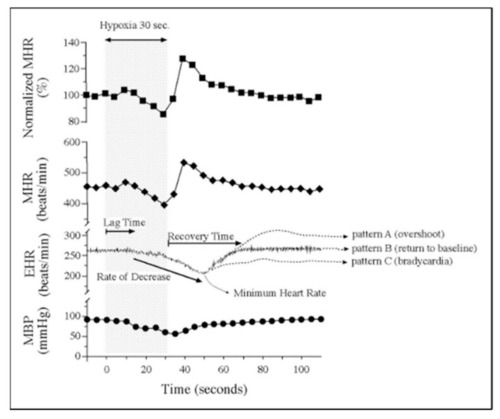
Embryonic vulnerability to acute maternal hypoxia. Representative maternal heart rate (MHR), embryonic heart rate (EHR), and maternal blood pressure (MBP) before, during, and after 30 s of maternal hypoxia via suspended ventilation. Lag time is defined as time from the onset of maternal hypoxia to the onset of bradycardia. Recovery time is defined as time from minimum EHR to return to baseline EHR. Patterns of EHR recovery are defined as ( |
|
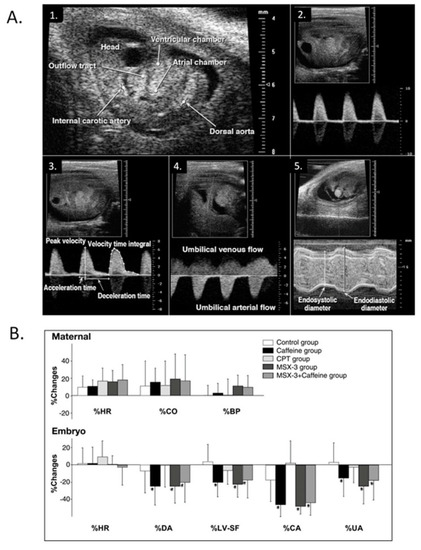
Modest maternal caffeine exposure affects murine embryonic cardiovascular function. ( |
|
Zebrafish embryo hemodynamics. ( |