- Title
-
Zebrafish xenografts as a fast screening platform for bevacizumab cancer therapy
- Authors
- Rebelo de Almeida, C., Mendes, R.V., Pezzarossa, A., Gago, J., Carvalho, C., Alves, A., Nunes, V., Brito, M.J., Cardoso, M.J., Ribeiro, J., Cardoso, F., Ferreira, M.G., Fior, R.
- Source
- Full text @ Commun Biol
|
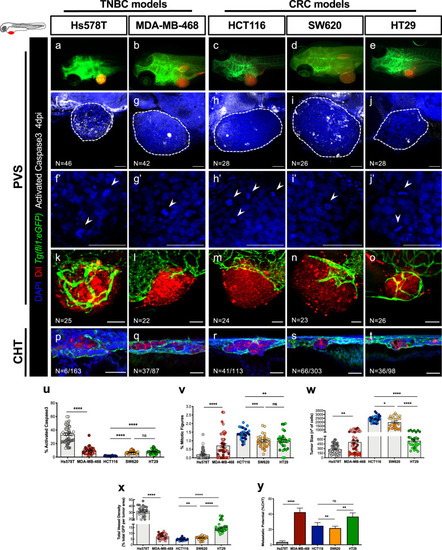
Human cancer cell lines (Hs578T, MDA-MB-468, HCT116, SW620 or HT29) were fluorescently labeled with DiI (red) and injected into the perivitelline space (PVS) of 2 days post fertilization (dpf) |
|
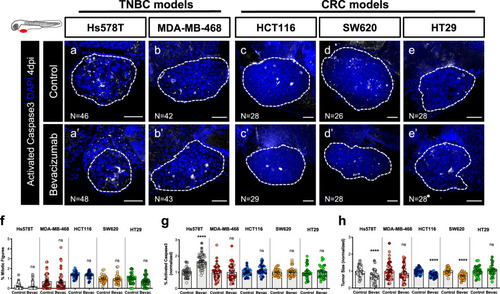
Human cancer cell lines (Hs578T, MDA-MB-468, HCT116, SW620 or HT29) were injected into the PVS of 2 dpf |
|
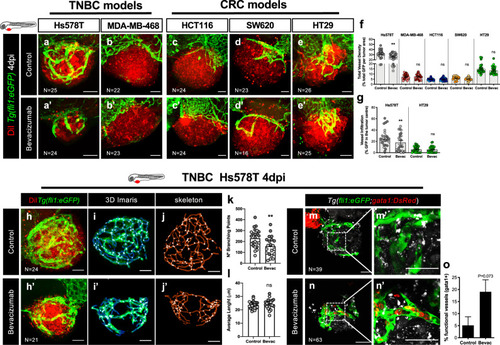
Human cancer cell lines (Hs578T, MDA-MB-468, HCT116, SW620 or HT29) were fluorescently labeled with DiI (in red) and injected into the PVS of 2 dpf |
|
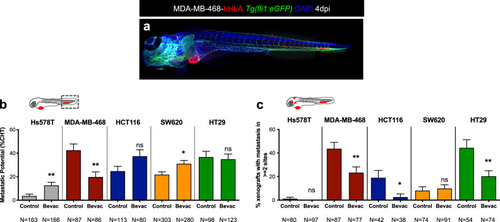
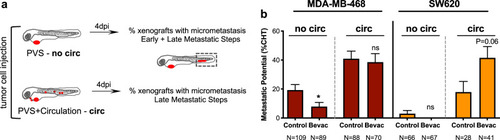
Representative image of an MDA-MB-468 zebrafish xenograft with a tumor in the PVS and several micrometastasis spread throughout the zebrafish larvae body, namely in brain, eye, gills and CHT ( |
|
Schematic representation to distinguish between early and late metastatic steps ( |
|
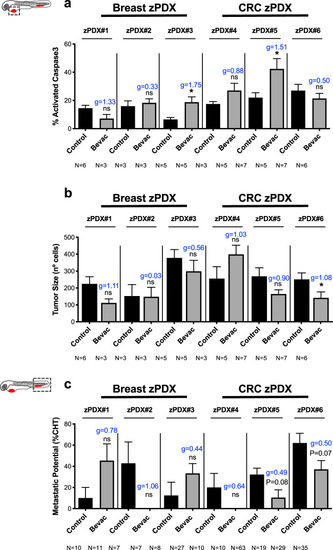
Human breast cancer or CRC surgical resected samples were injected into the PVS of 2 dpf |
|
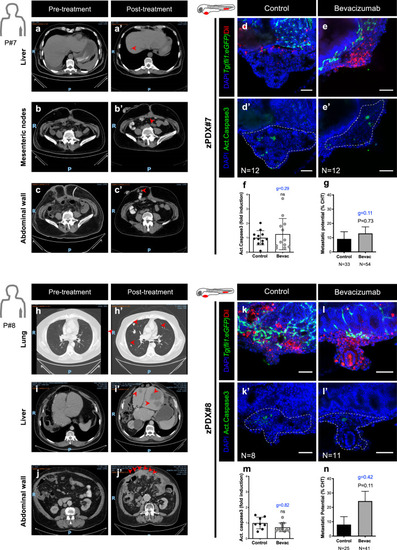
Computerized tomography scans of Patient#7 ( |