- Title
-
Reissner fibre-induced urotensin signalling from cerebrospinal fluid-contacting neurons prevents scoliosis of the vertebrate spine
- Authors
- Lu, H., Shagirova, A., Goggi, J.L., Yeo, H.L., Roy, S.
- Source
- Full text @ Biol. Open
|
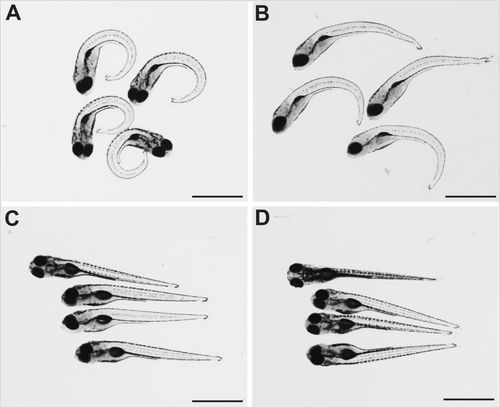
Dechorionation partly ameliorates the strong ventral curvature of the axis of sspo mutants. (A) sspo-mutant larvae that naturally hatched out of the chorion. (B) sspo-mutant larvae that were dechorionated at 24 hpf. (C) Wild-type sibling larvae that naturally hatched out of their chorion. (D) Wild-type sibling larvae that were dechorionated at 24 hpf. All larvae shown were imaged at 5 days post-fertilization (dpf) and are representative of 12 embryos analysed for each category. Scale bars: 1 mm. PHENOTYPE:
|
|
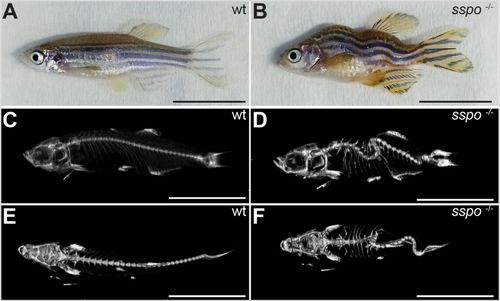
sspo mutants develop into adults with scoliotic spines. (A) A wild-type adult zebrafish. (B) An sspo mutant. Note the curved malformations of the trunk and tail. (C) MicroCT scan image of a wild-type zebrafish (lateral view). (D) MicroCT scan image of an sspo-mutant zebrafish (lateral view). Note the dorso-ventral curvatures of the spine. (E) MicroCT scan image of the wild-type zebrafish (dorsal view). (F) MicroCT scan image of the sspo-mutant zebrafish (dorsal view). Note the lateral curvatures of the spine. All fish were 3 months of age. Two fish were analysed for each genotype. Scale bars: 1 cm. PHENOTYPE:
|
|
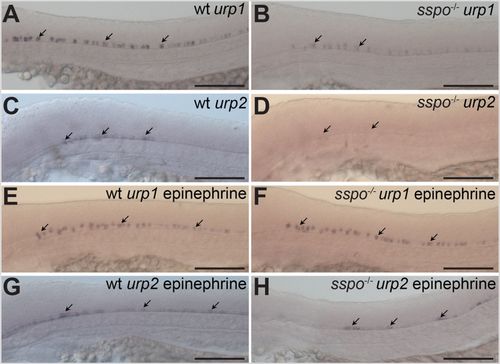
sspo mutants show loss of urp gene expression from CSF-cNs. (A) A wild-type embryo, showing urp1 expression in CSF-cNs (arrows). (B) An sspo-mutant embryo, showing reduction in levels of urp1 expression from CSF-cNs (arrows). (C) A wild-type embryo, showing urp2 expression in CSF-cNs (arrows). (D) An sspo-mutant embryo, showing almost complete lack of urp2 expression from CSF-cNs (arrows). Embryos shown in A–D are representative of a minimum of 25 embryos analysed for each genotype for each urp gene. (E) A wild-type embryo, showing urp1 expression in CSF-cNs after exposure to epinephrine (arrows). (F) An sspo-mutant embryo, showing urp1 expression in CSF-cNs after exposure to epinephrine (arrows). Note that expression level is indistinguishable from wild type. Embryos from mating of sspo-heterozygous fish were exposed to epinephrine in two independent experiments (25 embryos each). Since all embryos showed similar levels of urp1 expression, 13 embryos were randomly genotyped after in situ hybridisation from the second experiment, of which three were mutants. (G) A wild-type embryo, showing urp2 expression in CSF-cNs after exposure to epinephrine (arrows). (H) An sspo-mutant embryo, showing restoration of urp2 expression in CSF-cNs after exposure to epinephrine (arrows). Embryos from mating of sspo-heterozygous fish were exposed to epinephrine in two independent experiments (25 embryos each). 16 embryos from the second experiment, with urp2 expression in the spinal cord, were genotyped after in situ hybridisation, of which five were mutants. All embryos depicted in A–H were at 26 hpf. Scale bars: 100 μm. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
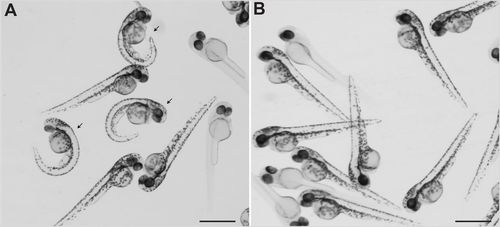
Exposure to epinephrine rescued axial curvature of sspo mutants. (A) sspo mutants (arrows) and siblings at 48 hpf. (B) sspo mutants and siblings at 48 hpf after epinephrine treatment. Note absence of embryos with curved bodies. Embryos shown in A and B are representative of a minimum of 100 embryos analysed in two independent experiments. Note, some embryos are devoid of body pigmentation as the sspo allele is maintained in the nacre mutant background that prevents body pigmentation in homozygotes. Scale bars: 1 mm. PHENOTYPE:
|
|
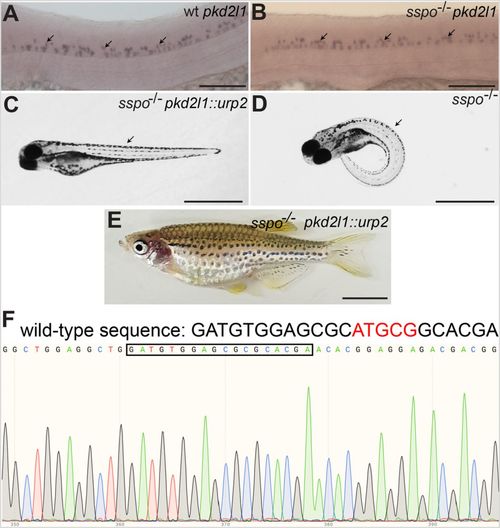
Axial defects of sspo mutants can be rescued by restoring urp2 expression specifically in CSF-cNs using the pkd2l1 promoter. (A) A wild-type embryo, showing pkd2l1 expression in CSF-cNs (arrows). (B) An sspo-mutant embryo showing normal levels and pattern of pkd2l1 expression in CSF-cNs (arrows). Embryos depicted in A and B were at 26 hpf and are representative of a minimum of 25 embryos analysed for each genotype. Scale bars: 100 μm. (C) An sspo-mutant larva rescued of axis curvature (arrow) by restoration of urp2 expression using the pkd2l1::urp2 transgene. A total of 150 embryos with straight axes from sspo/+ in-cross injected with pkd2l1::urp2 transgene from two independent experiments were genotyped, and 20 were found to be sspo-homozygous mutants. (D) An sspo-mutant larva. Note the strong ventrally curved axis (arrow). The larvae depicted were at 5 dpf. Scale bars: 1 mm. (E) An sspo-mutant adult rescued of its scoliotic spine by restoration of urp2 expression using the pkd2l1::urp2 transgene. Of 92 adults with straight axes, 31 were wild type, 60 were heterozygous and one was a homozygous mutant. Scale bar: 0.5 cm. (F) Electropherogram showing homozygous mutant genotype of the rescued sspo-mutant adult depicted in E. The wild-type sequence is indicated on top, with the five bp that are deleted in the mutant in red. The mutant sequence (with five bp deletion) is highlighted with the black box. |
|
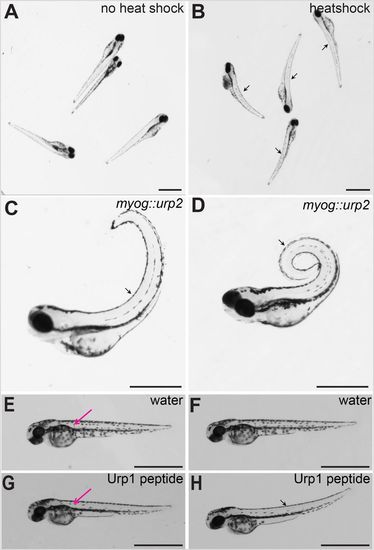
Urp over-expression causes dorsal curvature of the body axis in zebrafish embryos. (A) Wild-type embryos injected with hs::urp2 transgene, without heat induction. Note no effect on the body axis. (B) Same batch of embryos imaged after 30 min post-heat shock. Note the initiation of dorsal curvature of the body axis in the heat-shocked embryos (arrows). (C) A wild-type embryo at 72 hpf injected with myog::urp2 transgene, showing dorsal curvature of the trunk and tail (arrow). (D) A wild-type embryo at 5 dpf injected with myog::urp2 transgene showing spiral coiling of the body axis (arrow). Embryos shown in A–D are representative of a minimum of 100 embryos analysed for each condition of Urp2 over-expression, in two independent experiments. (E) A wild-type embryo at 48 hpf, injected with water in the dorsal somite, imaged immediately after injection. (F) The same wild-type embryo depicted in E, imaged after 1 h. Note no effect on body axis position. (G) A wild-type embryo at 48 hpf, injected with synthetic Urp1 in the dorsal somite, imaged immediately after injection. (H) The same wild-type embryo depicted in G, imaged after 5 min. Note the dorsal curvature of the body axis (arrow). In E and G, the injection sites are indicated (magenta arrows). Four embryos were injected for each condition. Scale bars: 1 mm. |
|
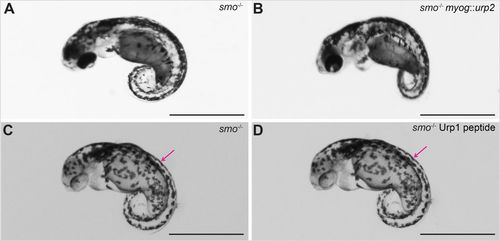
smo-mutant embryos, lacking slow-twitch fibres, are unresponsive to Urp over expression. (A) An smo-mutant embryo at 48 hpf. (B) An smo-mutant embryo injected with myog::urp2 transgene. Note that there was no effect on the ventrally curved axis. This experiment was performed in two independent biological replicates. In the first batch, five wild-type siblings showed no dorsal curvature and 57 showed dorsal curvature. All 12 smo mutants showed no response. In the second batch, of 105 wild-type siblings all showed dorsal curvature. All 24 smo mutants showed no response. (C) An smo-mutant embryo at 48 hpf, injected with water in the dorsal somite, imaged after 1 h. (D) An smo-mutant embryo, injected with Urp1 in the dorsal somite, imaged after 1 h. Note that there was no effect on the ventrally curved axis. The injection sites are indicated (magenta arrows). Four embryos were injected for each condition. Scale bars: 1 mm. PHENOTYPE:
|