- Title
-
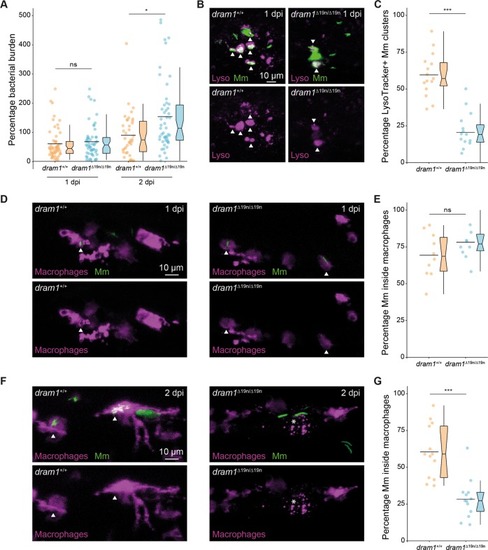
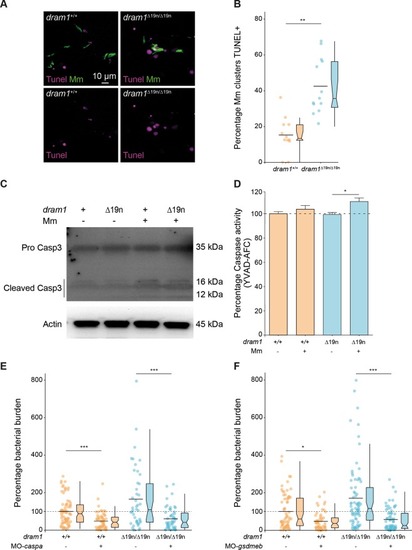
Deficiency in the autophagy modulator Dram1 exacerbates pyroptotic cell death of Mycobacteria-infected macrophages
- Authors
- Zhang, R., Varela, M., Forn-Cuní, G., Torraca, V., van der Vaart, M., Meijer, A.H.
- Source
- Full text @ Cell Death Dis.
|
PHENOTYPE:
|
|
|
|
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|