- Title
-
Type 1 Interleukin-4 Signaling Obliterates Mouse Astroglia in vivo but Not in vitro
- Authors
- Mashkaryan, V., Siddiqui, T., Popova, S., Cosacak, M.I., Bhattarai, P., Brandt, K., Govindarajan, N., Petzold, A., Reinhardt, S., Dahl, A., Lefort, R., Kizil, C.
- Source
- Full text @ Front Cell Dev Biol
|
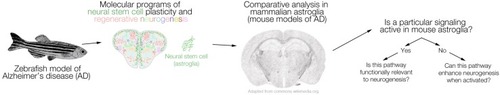
A schematic representation of the comparative logic between zebrafish and mammalian models of Alzheimer’s disease (AD). The regenerative neurogenic ability of zebrafish brain can be harnessed to elucidate the mechanisms, which can be further tested in mouse models of AD. If a particular signaling that enables regeneration in zebrafish brain is active also in mouse brains (e.g., in neural stem cells or progenitors), functional relevance to neurogenesis can be tested. If such a program ceases to exist in mammalian brains, activation of the pathway or its components can be tested as to whether they induce the regenerative neurogenic ability in mammalian brains. We propose this workflow as a stringent comparative analysis pipeline between zebrafish and mammalian brains. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
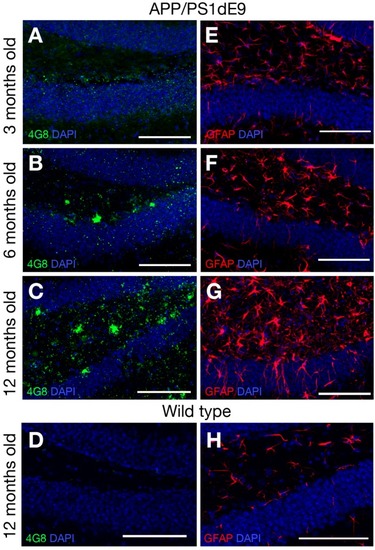
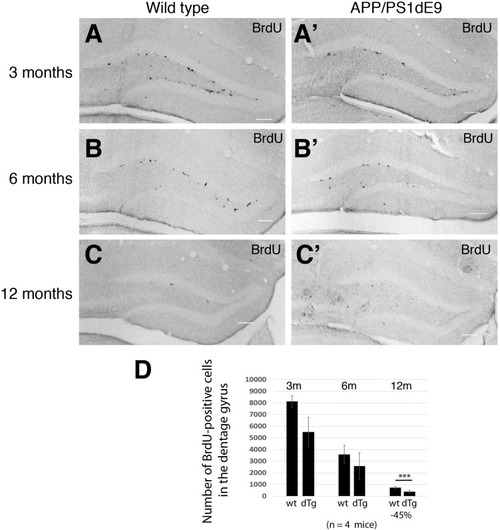
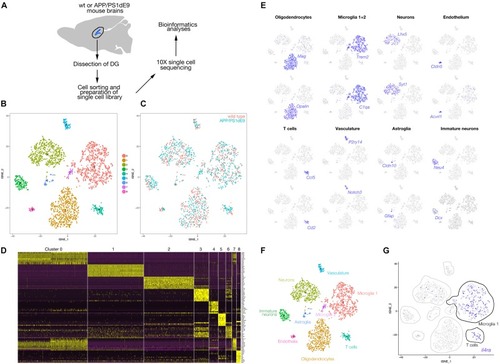
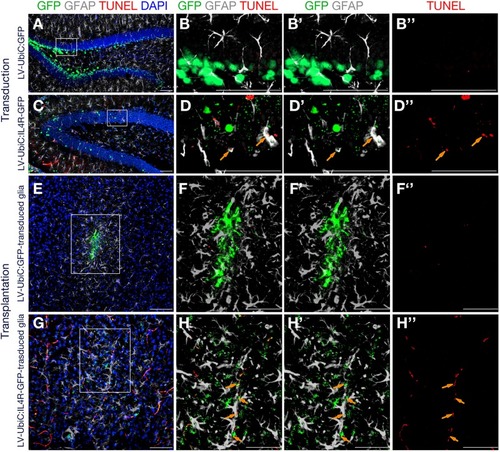
Immunostaining for GFAP (gray) and GFP (green) coupled to TUNEL staining (red). |