- Title
-
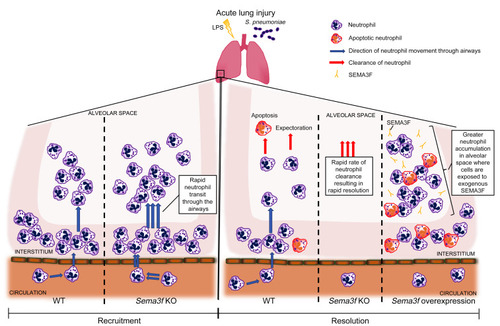
Semaphorin 3F signaling actively retains neutrophils at sites of inflammation
- Authors
- Plant, T., Eamsamarng, S., Sanchez-Garcia, M.A., Reyes, L., Renshaw, S.A., Coelho, P., Mirchandani, A.S., Morgan, J.M., Ellett, F.E., Morrison, T., Humphries, D., Watts, E.R., Murphy, F., Raffo-Iraolagoitia, X.L., Zhang, A., Cash, J.L., Loynes, C., Elks, P.M., Van Eeden, F., Carlin, L.M., Furley, A.J.W., Whyte, M.K.B., Walmsley, S.R.
- Source
- Full text @ Journal of Clin. Invest.
|
( |
|
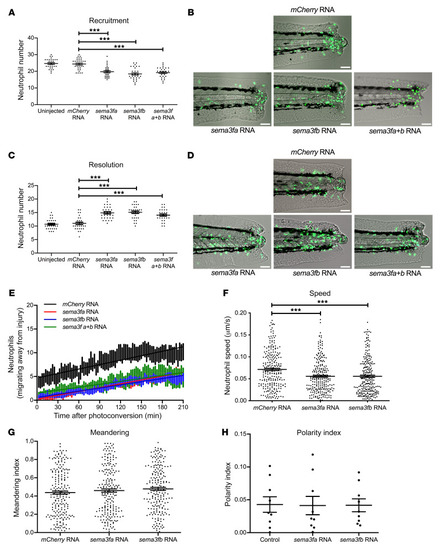
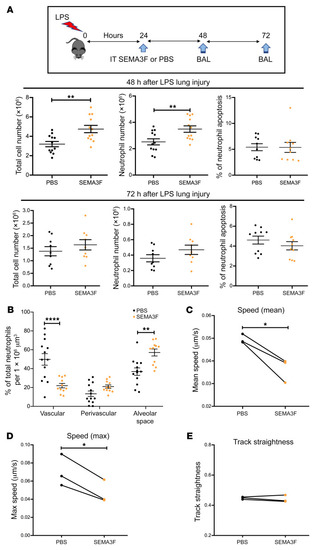
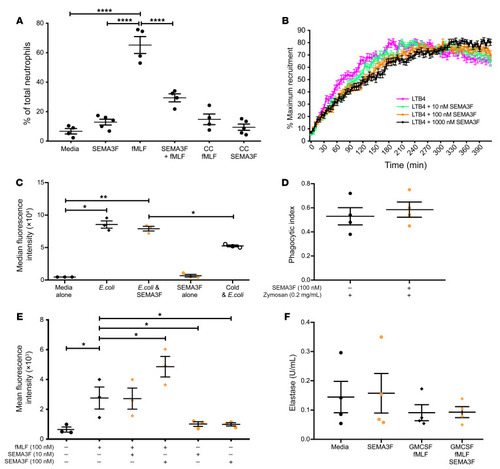
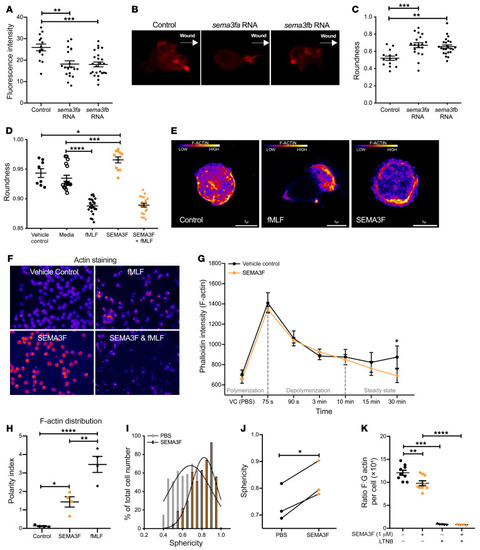
( PHENOTYPE:
|
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
Neutrophil-specific KO of |