- Title
-
Recellularized Colorectal Cancer Patient-derived Scaffolds as in vitro Pre-clinical 3D Model for Drug Screening
- Authors
- Sensi, F., D'Angelo, E., Piccoli, M., Pavan, P., Mastrotto, F., Caliceti, P., Biccari, A., Corallo, D., Urbani, L., Fassan, M., Spolverato, G., Riello, P., Pucciarelli, S., Agostini, M.
- Source
- Full text @ Cancers
|
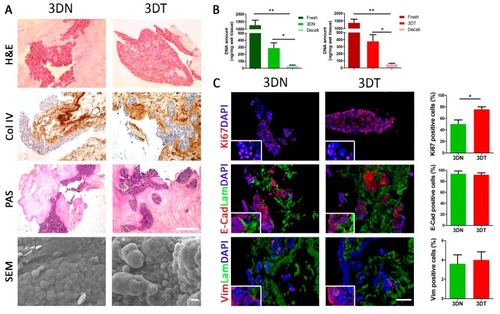
Characterization of matched 3DN and 3DT HT29-recellularized samples. ( |
|
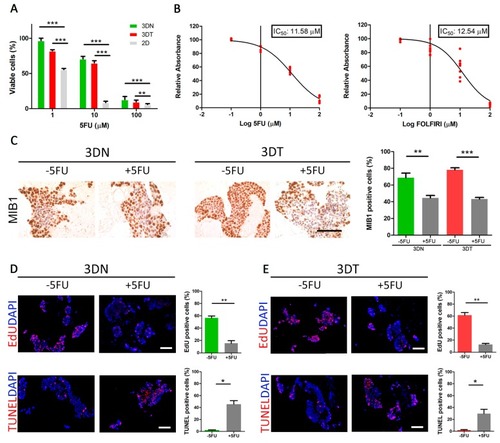
Effect of 5-fluorouracil (5FU) and FOLFIRI ((leucovorin + 5-fluorouracil (5FU) + irinotecan) treatments on HT29-cells cultured in a 3D model. ( |
|
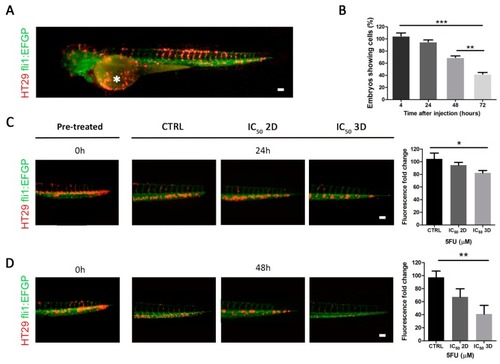
Effect of 5FU treatment on in vivo zebrafish model. ( EXPRESSION / LABELING:
|
|
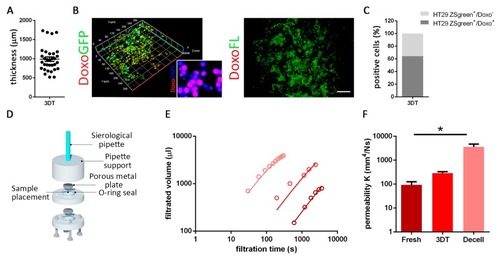
Drug diffusion evaluation. ( |