- Title
-
The evolution of zebrafish RAG2 protein is required for adapting to the elevated body temperature of the higher endothermic vertebrates
- Authors
- Sun, A., Xu, K., Liu, H., Li, H., Shi, Y., Zhu, X., Liang, T., Li, X., Cao, X., Ji, Y., Jiang, T., Xu, C., Liu, X.
- Source
- Full text @ Sci. Rep.
|
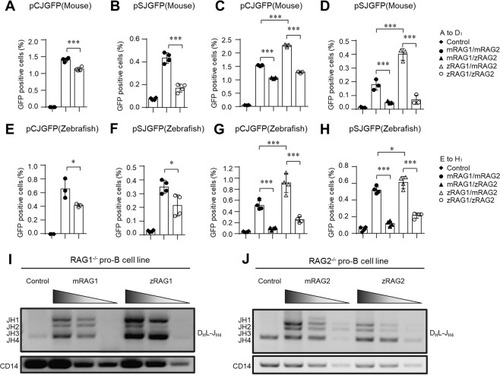
The recombination efficiency of zRAG is lower than that of mRAG at 37 °C. NIH3T3 cells were co-transfected with pCJGFP (mouse) ( |
|
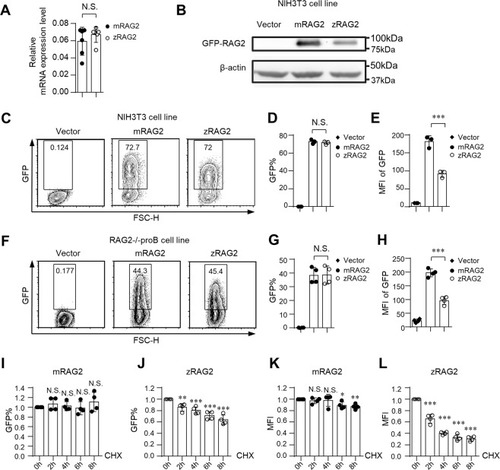
The zRAG2 protein is unstable compared to the mRAG2 protein at 37 °C. ( |
|
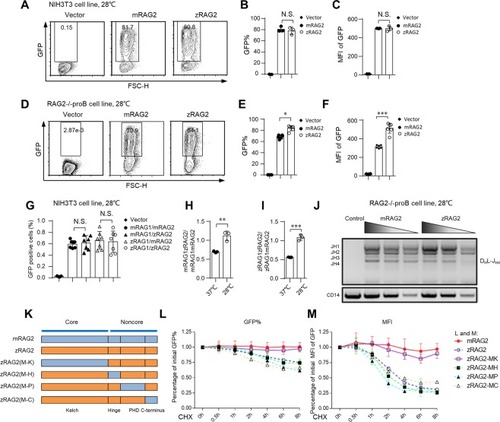
The protein stability and recombination efficiency of zRAG2 are similar to those of mRAG2 at 28 °C. ( |
|
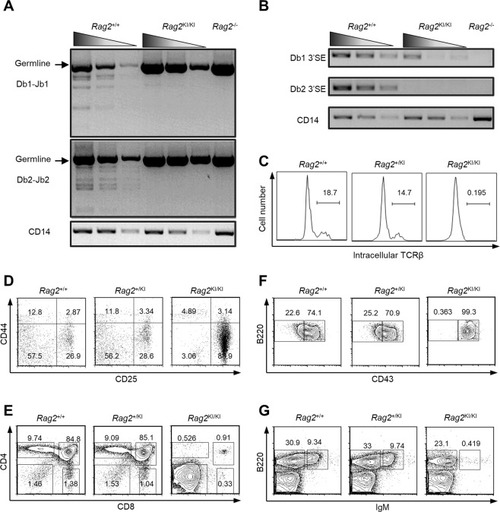
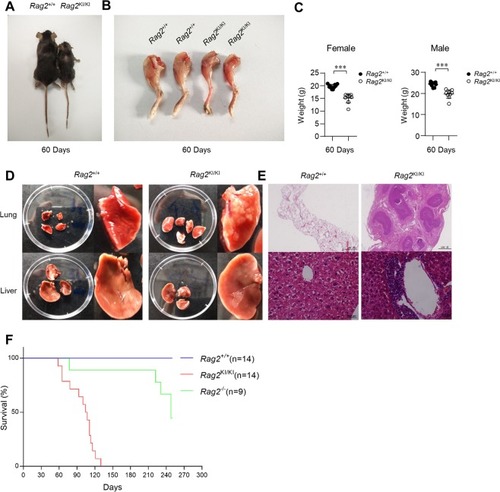
The V(D)J recombination of |
|
|
|
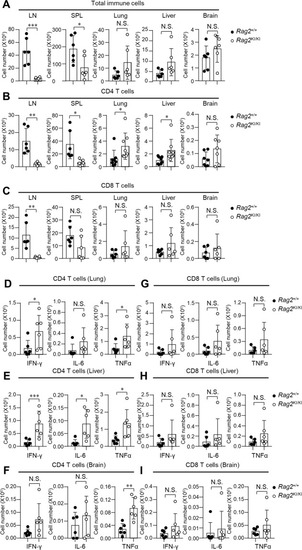
T cells that produced cachexia cytokines of |