- Title
-
Generation and Characterization of a CRISPR/Cas9 -Induced 3-mst Deficient Zebrafish
- Authors
- Katsouda, A., Peleli, M., Asimakopoulou, A., Papapetropoulos, A., Beis, D.
- Source
- Full text @ Biomolecules
|
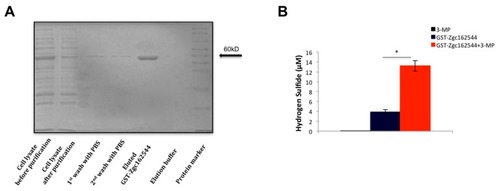
Purification and enzymatic activity of recombinant GST-Zgc162544. ( |
|
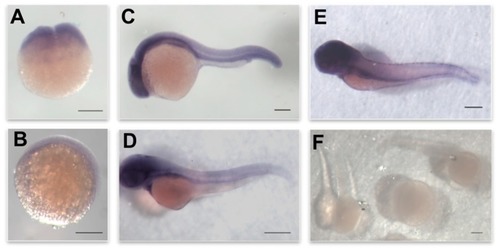
Expression pattern of EXPRESSION / LABELING:
|
|
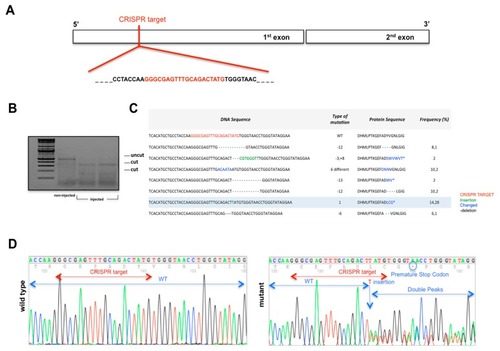
Generation of |
|
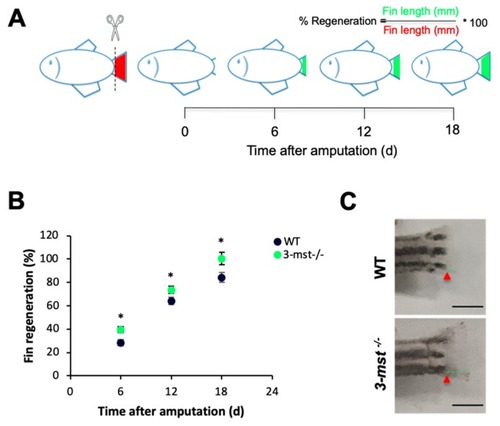
PHENOTYPE:
|
|
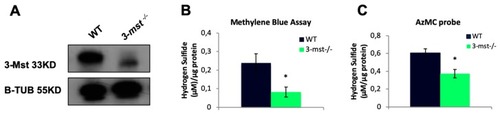
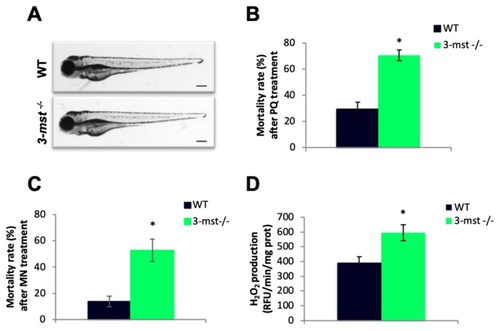
Increased H2O2 production and oxidative stress sensitivity in |
|
PHENOTYPE:
|