- Title
-
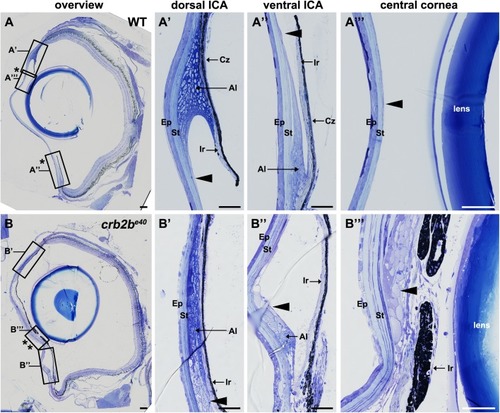
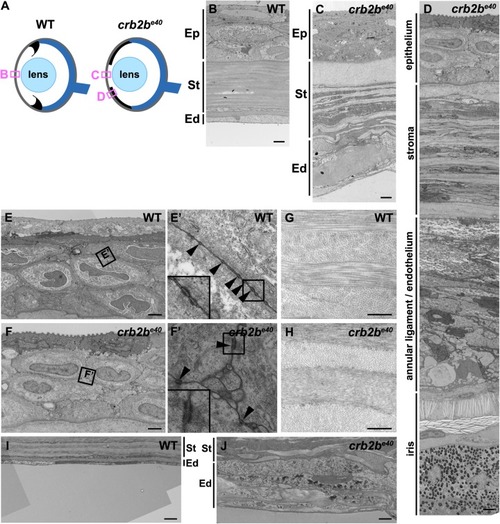
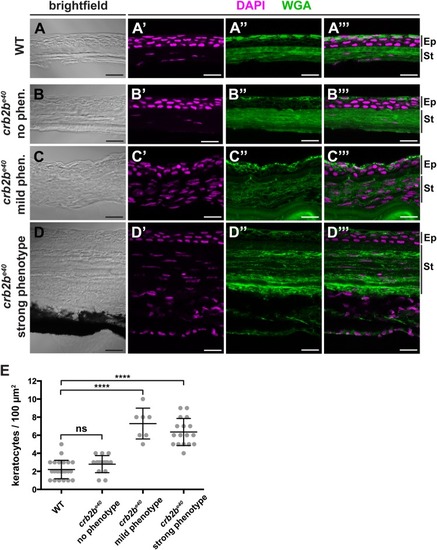
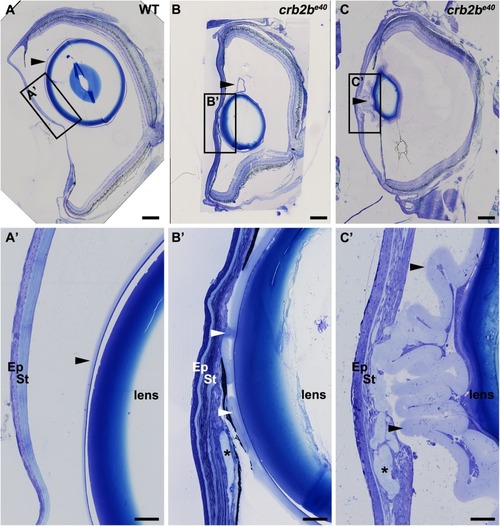
Loss of Crb2b-lf leads to anterior segment defects in old zebrafish
- Authors
- Kujawski, S., Crespo, C., Luz, M., Yuan, M., Winkler, S., Knust, E.
- Source
- Full text @ Biol. Open
|
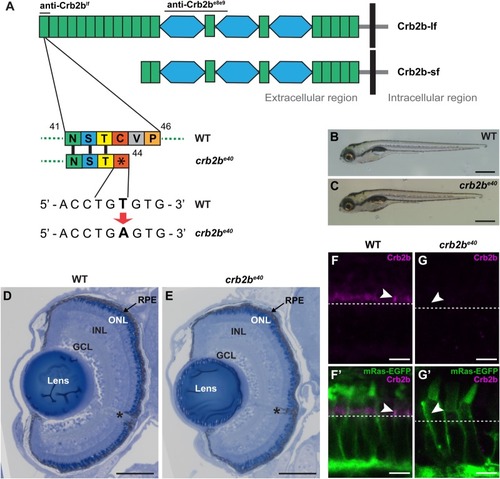
EXPRESSION / LABELING:
PHENOTYPE:
|
|
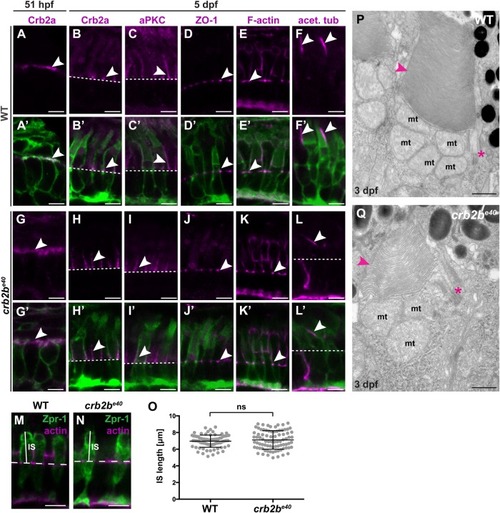
PHENOTYPE:
|
|
|
|
PHENOTYPE:
|
|
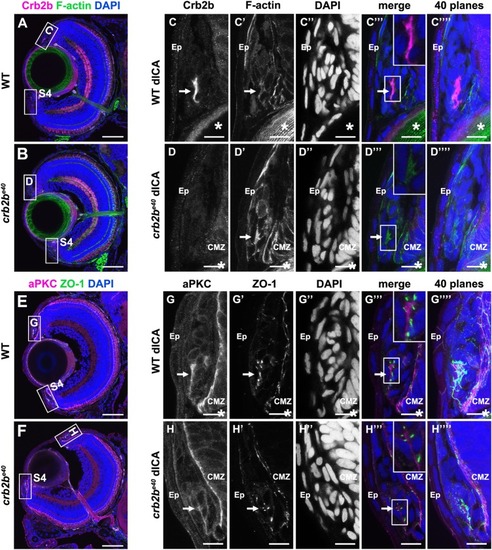
PHENOTYPE:
|
|
PHENOTYPE:
|
|
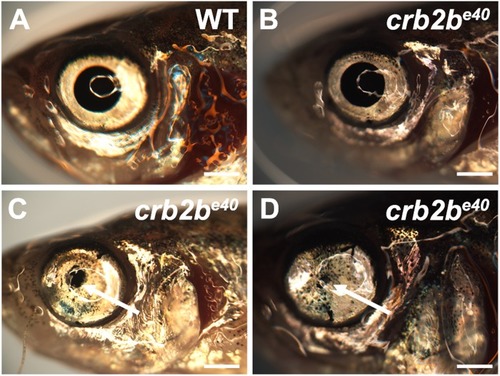
PHENOTYPE:
|
|
PHENOTYPE:
|