- Title
-
The Zebrafish Xenograft Platform-A Novel Tool for Modeling KSHV-Associated Diseases
- Authors
- Pringle, E.S., Wertman, J., Melong, N., Coombs, A.J., Young, A.L., O'Leary, D., Veinotte, C., Robinson, C.A., Ha, M.N., Dellaire, G., Druley, T.E., McCormick, C., Berman, J.N.
- Source
- Full text @ Viruses
|
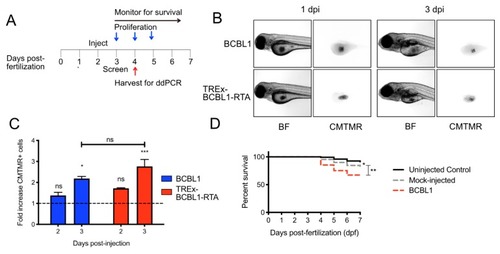
Proliferation of BCBL and TREx-BCBL1-RTA in zebrafish larvae: ( |
|
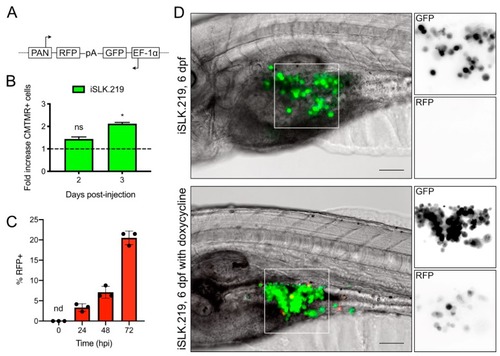
Proliferation and lytic reactivation of iSLK.219 in zebrafish embryos: ( |
|
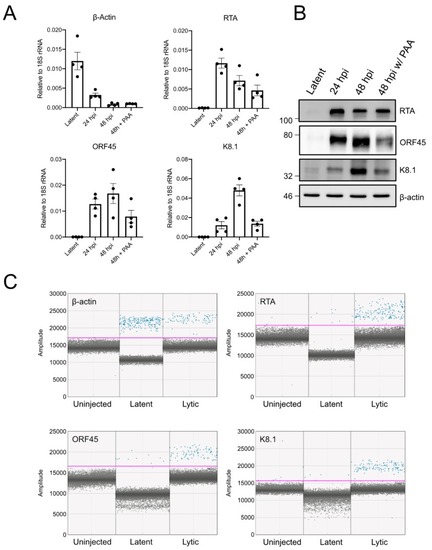
Detection of viral gene expression in xenotransplanted cells by ddPCR: ( |
|
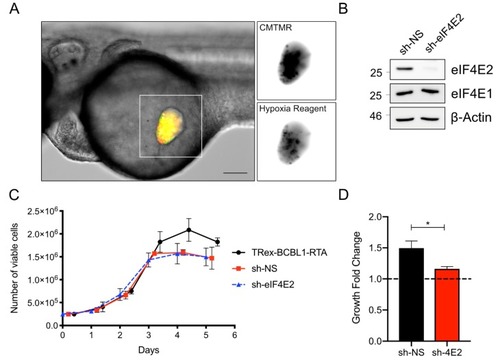
The zebrafish yolk sac is hypoxic and xenotransplant proliferation requires eIF4E2: ( |