- Title
-
Characterizing renal involvement in Hermansky-Pudlak Syndrome in a zebrafish model
- Authors
- Schenk, H., Müller-Deile, J., Schroder, P., Bolaños-Palmieri, P., Beverly-Staggs, L., White, R., Bräsen, J.H., Haller, H., Schiffer, M.
- Source
- Full text @ Sci. Rep.
|
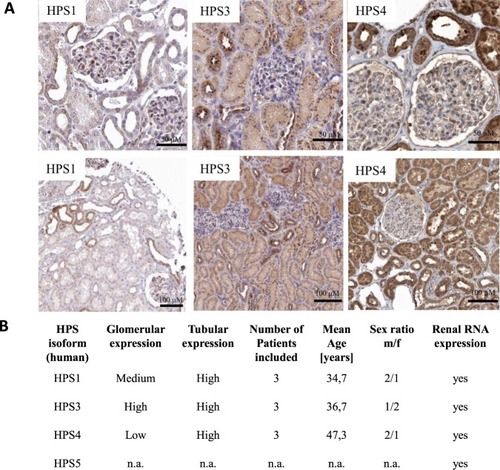
Cellular expression of HPS proteins was analyzed by immunohistochemistry in human kidney sections. ( |
|
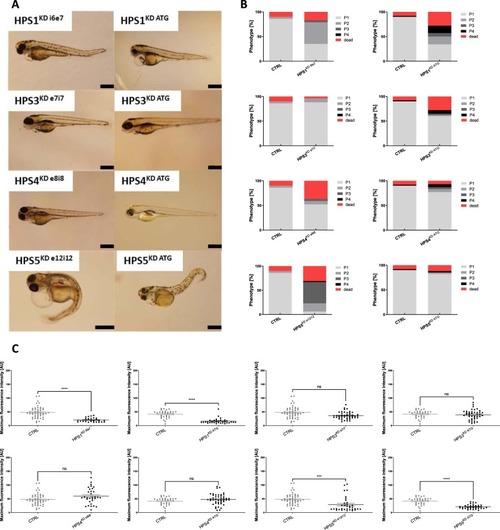
Phenotype evaluation and renal protein loss determination after induction of HPS1KD, HPS3KD, HPS4KD and HPS5KD in zebrafish. ( PHENOTYPE:
|
|
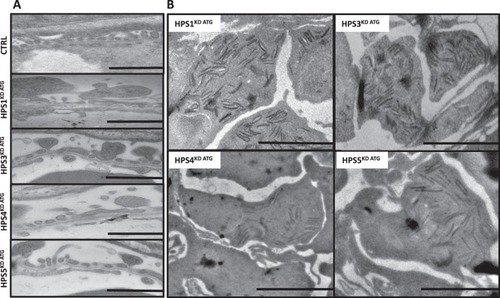
HPS1KD ATG, HPS3KD ATG, HPS4KD ATG and HPS5KD ATG induce varying degrees of glomerular damage including podocyte effacement and deposition of intracellular debris (zebra bodies). Transmission electron microscopy imaging was used to detect ultrastructural changes in glomeruli after HPS protein knockdown induced by MO injection in PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|