- Title
-
Inhibition of the thyroid hormonogenic H2O2 production by Duox/DuoxA in zebrafish reveals VAS2870 as a new goitrogenic compound
- Authors
- Giusti, N., Gillotay, P., Trubiroha, A., Opitz, R., Dumont, J.E., Costagliola, S., De Deken, X.
- Source
- Full text @ Mol. Cell. Endocrinol.
|
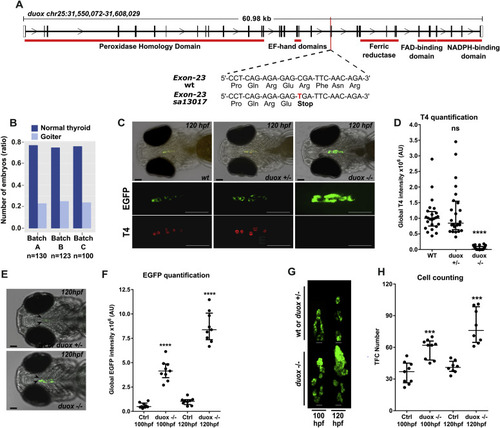
The duox sa13017 mutant larvae develop dyshormonogenic goiter phenotype (A) Chromosome 25 genomic locus with duox sequences for wild-type (WT) and mutant (duox sa13017) alleles. A single nucleotide exchange (C–T) in duox gene generates a stop codon in the exon 23 after the glutamate 997 and upstream of the regions encoding the functional domains of the protein. (B) Proportion of larvae (120 hpf) presenting a goitrous thyroid phenotype as detected in the progeny from three independent mating experiments of heterozygous duox sa13017 fish. (C) Whole-mount immunofluorescence (WIF) staining for EGFP and T4 of 120 hpf duox sa13017 mutants showed strong reduction of detectable T4 signal and thyroid enlargement in all homozygous (−/−) mutant larvae compared to WT and heterozygous (+/-) larvae. Epifluorescence images are shown (ventral views, anterior to the left, scale bars: 75 μm). (D) Quantification of colloidal T4 immunofluorescence signals. Data are presented in a scatter plot with the median and the interquartile ranges, dots representing the total follicular T4 intensity of individual fish. Eight specimens per genotype were analyzed from three independent experiments. No statistical difference (ns) was observed between WT and heterozygous (+/-) duox sa13017 mutants. (E–F) Epifluorescence live imaging analysis of 100 and 120 hpf duox sa13017 larvae. Panel E shows representative images of the thyroid region in heterozygous and homozygous mutant duox sa13017 fish at 120 hpf (ventral view of the head, anterior to the left, scale bars: 75 μm). Arrow heads point to the thyroidal EGFP reporter signal. Panel F shows the quantification of EGFP signals in 100 and 120 hpf larvae. Scatter plots show the median with the interquartile ranges. Dots represent the global EGFP intensity of individual fish (n = 9). Ctrl: WT or heterozygous duox sa13017 mutant larvae. (G) Confocal live imaging of duox sa13017 larvae (100 and 120 hpf). 3D reconstruction of thyroid tissue shows a clear thyroid enlargement associated with the homozygous duox sa13017 mutation at 120 hpf (ventral view, anterior to the top). Scale bars: 20 μm. (H) Quantification of thyroid follicular cell (TFC) number in WT or heterozygous duox mutants (Ctrl) and homozygous duox sa13017 (−/−) mutants. Scatter plots show the median with the interquartile ranges. Each dot represents the total cell number of individual fish (n = 7–9). Mann-Whitney tests were performed to compare duox (−/−) mutants with their respective 100 or 120 hpf control specimens. |
|
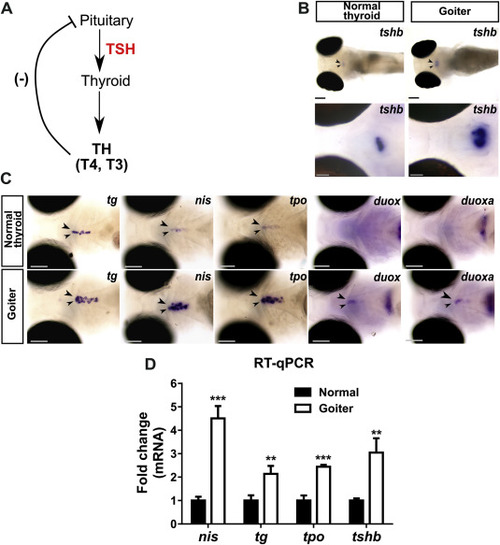
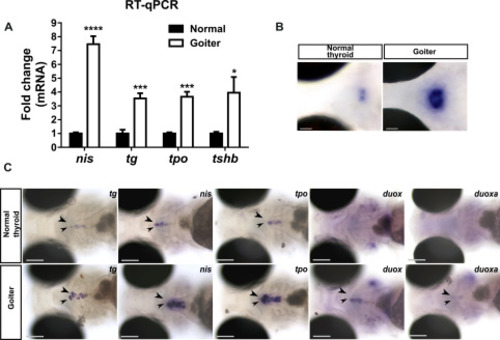
Overstimulation of the pituitary-thyroid axis in 120 hpf hypothyroid duox sa13017 mutant larvae (A) Schematic representation of the pituitary-thyroid axis with the thyroid hormones (TH)-mediated negative feedback loop inhibiting thyrotropin (TSH) secretion. (B) Whole-mount in situ hybridization (WISH) showed that larvae presenting a goitrous phenotype have increased tshb mRNA expression in the pituitary gland (arrow heads) compared to unaffected siblings. Images at the top show dorsal views of whole embryos (anterior to the left, scale bars: 100 μm). Images at the bottom show higher magnification views of the pituitary in coronal sections (anterior to the left, scale bars: 50 μm). (C) At the thyroid level (arrow heads), increased expression of TSH-dependent functional genes (tg, slc5a5/nis, tpo, duox and duoxa) was detected by WISH in larvae with a goitrous thyroid. Head region of representative larvae are shown in ventral views (anterior to the left, scale bars: 100 μm). (D) Fold change (mean ± SD, n = 3) of tg, slc5a5/nis, tpo and tshb mRNA expression in goiter affected animals compared to normal (WT or heterozygous mutants) specimens. |
|
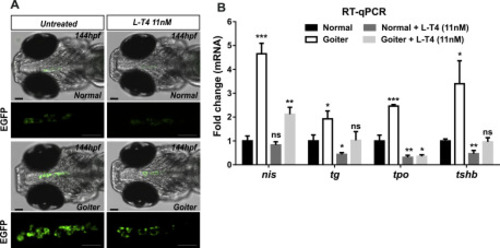
L-T4 supplementation of duox sa13017 mutant larvae. The progeny of heterozygous duox sa13017 fish (Tg(tg:nlsEGFP) background) were selected based on thyroid phenotypes at 100 hpf and corresponding larvae were treated or not with 11 nM L-thyroxine (L-T4) until 144 hpf to be analyzed. (A) Epifluorescence live imaging of thyroidal EGFP signal. Representative images (ventral view of the head region, anterior to the left, scale bars: 75 μm) showing a marked decrease of the EFGP signal in L-T4 treated animals. (B) Comparative fold change mRNA expression of tg, slc5a5/nis, tpo and tshb genes compared to normal (WT or heterozygous mutants) non-treated animals. The means with SD are presented on the graphs (n = 3). Mann-Whitney tests were performed to compare normal non-treated larvae with all other conditions. PHENOTYPE:
|
|
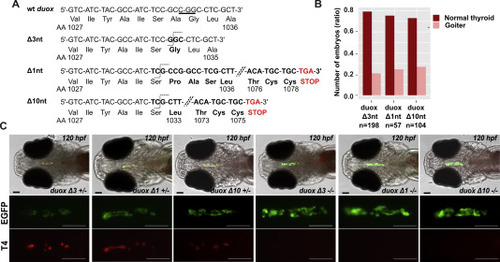
duox mutant fish are affected by thyroid dyshormonogenesis. (A) Target site for duox gRNA located in the exon 23 (CGG PAM sequence is underlined). The duox mutated sequences of the three mutant fish lines presenting deletions of 1, 3 or 10 nucleotides are shown. Frame-shift mutations in duox Δ1nt and Δ10nt mutant lines generate premature stop codons after residues 1078 and 1075, respectively. (B) Proportion of larvae (120 hpf) presenting a goitrous thyroid phenotype as detected in the progeny of mating experiments of heterozygous duox Δ1nt, Δ3nt and Δ10nt mutant fish, respectively. (C) WIF staining for thyroid EGFP reporter and colloidal T4 of 120 hpf duox Tg(tg:nlsEGFP) mutants showed important reduction of detectable T4 signal and thyroid enlargement in all homozygous (−/−) duox mutant larvae (eight specimens per genotype analyzed). Representative epifluorescence images are shown (ventral views, anterior to the left, scale bars: 75 μm). |
|
Overstimulation of the pituitary-thyroid axis in 120 hpf hypothyroid duoxa mutant larvae. Heterozygous duoxa Δ11nt mutant fish (in Tg(tg:nlsEGFP) background) were mated and the progeny raised to 120 hpf for live imaging. Larvae were sorted according to thyroid phenotype (normal or goiter). (A) Fold change (mean ± SD, n = 3) of tg, slc5a5/nis, tpo and tshb mRNA expression in goiter affected animals compared to normal (WT or heterozygous mutants) specimens. (B) Increase of tshb mRNA expression by WISH in the pituitary gland of larvae presenting a goitrous phenotype compared to unaffected siblings. Representative coronal sections are shown (dorsal view, anterior to the left, scale bars: 50 μm). (C) At the thyroid level, increased expression of TSH-dependent functional genes (tg, slc5a5/nis, tpo, duox and duoxa) was detected (arrow heads) by WISH in larvae with a goitrous thyroid. Representative larvae are shown in ventral views of the head region (anterior to the left). Scale bars: 100 μm. |
|
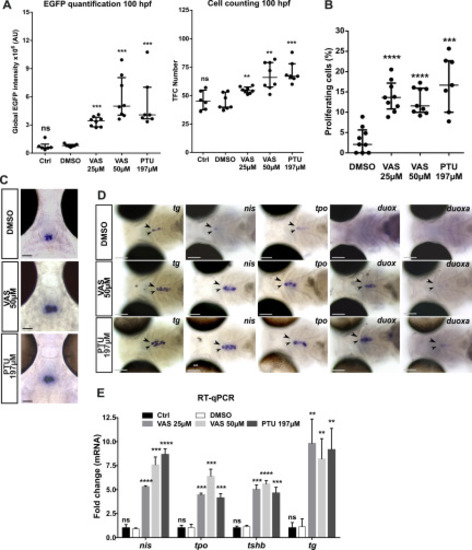
Effects of the goitrogenic compound VAS2870 on thyroid differentiation marker expression and cell proliferation. Tg(tg:nlsEGFP) larvae were treated with VAS2870 (25, 50 μM) from 52 to 120 hpf. Negative controls included embryo medium (Ctrl) and 0.025% DMSO (vehicle). Treatment with 197 μM PTU served as a positive control. (A) Live imaging of EGFP reporter expression was performed for quantification of thyroidal EGFP reporter signal (epifluorescence analyzes) and total TFC number (confocal analyzes) in 100 hpf larvae. Scatter plots show the median with the interquartile ranges. Each dot represents the global EGFP intensity or the total cell number for individual fish. Each treatment was replicated in three independent experiments with minimum three embryos per condition analyzed. (B) Percentage of thyroid proliferating cells (number of EdU+/EGFP+ cells divided by the TFC number (DAPI+/EFGP+)) in treated or vehicle control (DMSO) 120 hpf Tg(tg:nlsEGFP) larvae. Scatter plots show the median with the interquartile ranges. Each dot represents the % of proliferating cells of individual fish (n=7-9). (C) Pituitary tshb mRNA expression detected by WISH in 120 hpf treated larvae from 52 hpf. Representative coronal sections are shown (dorsal view, anterior to the top, scale bars: 50 μm). (D) At the thyroid level (arrow heads), increased expression of TSH-dependent functional markers (tg, slc5a5/nis, tpo, duox and duoxa) was detected in 120 hpf treated larvae. Representative larvae are shown in ventral views of the head region (anterior to the left). Scale bars: 100 μm. (E) Comparative fold change mRNA expression (mean ± SD, n=3) of tg, slc5a5/nis, tpo and tshb genes using DMSO treated larvae as controls. Mann-Whitney tests were performed to compare DMSO treated larvae with all other conditions. |
Reprinted from Molecular and Cellular Endocrinology, 500, Giusti, N., Gillotay, P., Trubiroha, A., Opitz, R., Dumont, J.E., Costagliola, S., De Deken, X., Inhibition of the thyroid hormonogenic H2O2 production by Duox/DuoxA in zebrafish reveals VAS2870 as a new goitrogenic compound, 110635, Copyright (2019) with permission from Elsevier. Full text @ Mol. Cell. Endocrinol.