- Title
-
Prepontine non-giant neurons drive flexible escape behavior in zebrafish
- Authors
- Marquart, G.D., Tabor, K.M., Bergeron, S.A., Briggman, K.L., Burgess, H.A.
- Source
- Full text @ PLoS Biol.
|
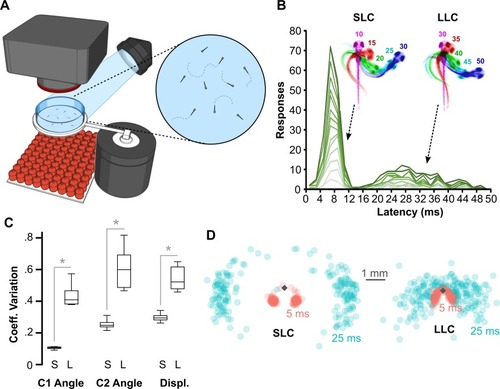
(A) Schematic of behavioral experiments in free-swimming larvae: groups of 15–20 6-dpf larvae were imaged from above at 1,000 frames per second with a high-speed camera. An infrared LED array below provided illumination. Nondirectional acoustic/vibratory stimuli were delivered to the arena by a minishaker. (B) Frequency histogram of response latencies for individual larvae ( |
|
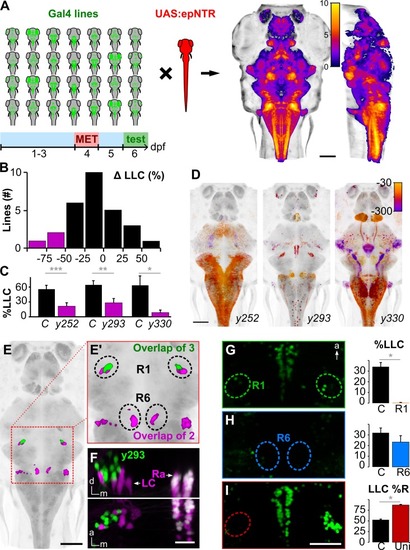
(A) Schematic of circuit-breaking screen: 28 Gal4 enhancer trap lines were crossed to |
|
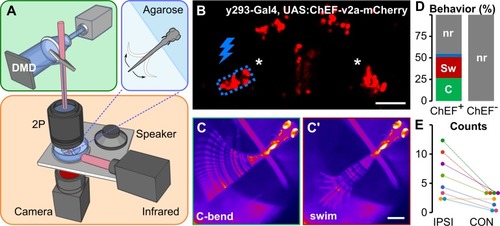
(A) Schematic of optogenetic stimulation and two-photon calcium imaging: a DMD was used to spatially restrict 460-nm LED excitation (green box) within the brain of head-embedded larvae (blue box) mounted on a stage with a speaker for acoustic/vibratory stimulation, an infrared light source for tail illumination, and a high-speed camera for behavioral readout (orange box). (B) Two-photon optical section of mCherry expression in |
|
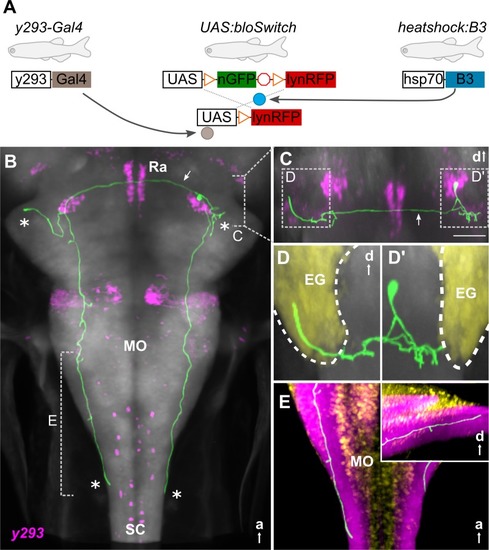
(A) Schematic of 3 transgene system used for B3-recombinase–based neuronal tracing. (B-E) Representative traced neuron in 6-dpf larva (green, for others, see |
|
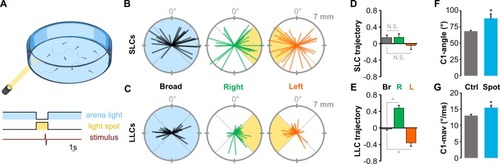
(A) Schematic of experiment measuring escape direction under broad-field illumination or in darkness with only a light spot illuminated. Scale bar 1 s. (B-C) Thirty representative SLC (B) and LLC (C) escape trajectories of larvae to a nondirectional acoustic/vibratory stimulus when under broad-field illumination (“Broad”) or when oriented to the left or to the right of a light spot. Escape direction is plotted radially and net displacement axially. (D-E) Mean direction choice (−1 all left; +1 all right) for SLC (D) and LLC (E) responses under broad-field illumination (“Br”; SLC, |
|
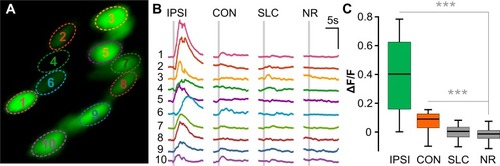
(A) Two-photon optical section of nuclear-localized GCaMP6s-positive prepontine neurons in |
|
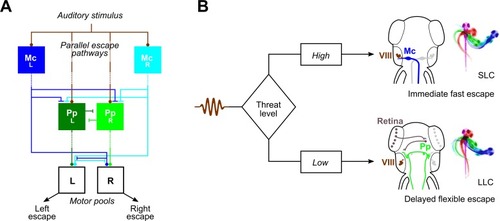
(A) Parallel sensory pathways transmit acoustic information to M-cells (“McL” and “McR”) and prepontine escape neurons (“PpL” and “PpR”). Decision-making is based on reaction time: VIIIth nerve activation of M-cells is direct, whereas prepontine neurons receive auditory information only via an indirect pathway, allowing active M-cells to prevent the initiation of delayed escapes. Additionally, Mauthner cells inhibit prepontine escape neurons, likely through an indirect pathway. Dashed lines indicate indirect connections. (B) Anatomy corresponding to the model in (A). Auditory signals from the statoacoustic ganglion (VIII, brown) excite M-cells (“Mc,” blue) directly (top) or prepontine escape neurons (“Pp,” green) indirectly (bottom). M-cells receive predominantly ipsilateral inputs and project commissurally to drive fast escapes, whereas prepontine escape neurons project both ipsi- and contralaterally and may drive escape in either direction, although predominantly ipsilaterally. LLC, long-latency C-start; M-cell, Mauthner cell; SLC, short-latency C-start. |