- Title
-
Frizzled 4 regulates ventral blood vessel remodeling in the zebrafish retina
- Authors
- Caceres, L., Prykhozhij, S.V., Cairns, E., Gjerde, H., Duff, N.M., Collett, K., Ngo, M., Nasrallah, G.K., McMaster, C.R., Litvak, M., Robitaille, J.M., Berman, J.N.
- Source
- Full text @ Dev. Dyn.
|
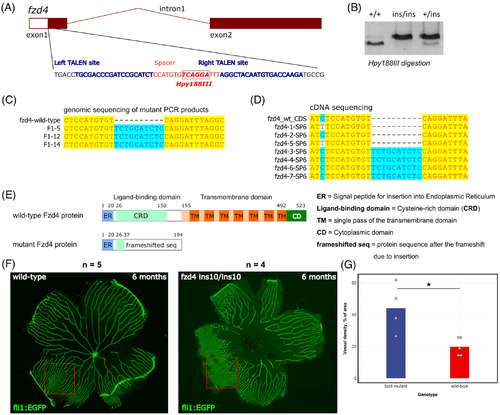
Generation of the fzd4 ins10 mutant and initial characterization of its retinal vasculature phenotype. A, The exon‐intron structure of the zebrafish fzd4 gene is shown with a zoomed‐in section of exon 1, which was targeted by TALENs. The targeted part of exon 1 contains left and right binding sites for TALEN proteins and the spacer sequence between them is the mutagenesis target. The site of the Hpy188III enzyme within the spacer is indicated by a label, box, and bold font. B, An example of the 10‐nucleotide insertion mutant (ins10) genotyping by first amplifying PCR products of the target site from wild‐type (+/+), heterozygous (+/ins) and homozygous mutant (ins/ins) animals followed by Hpy188III digestion and agarose gel electrophoresis. C, Sequencing results of the Hpy188III‐undigestable PCR products derived from F1 mutation‐carrier zebrafish show clear evidence of the 10‐nucleotide insertion at the TALEN‐targeted site. D, Sequencing of individual plasmid clones containing cDNA fragments from the RNA of fzd4 +/ins10 incrossed embryos. Among eight clones analyzed, one was empty (not shown), four had the insertion, and three had the wild‐type version of the cDNA. E. Domain diagrams and sizes are shown for the wild‐type (523 amino acids) and ins10 mutant (194 amino acids in total, with 37 N‐terminal amino acids of Fzd4). Fzd4 proteins predicted from cDNA sequencing. The following domains and structures are shown: ER = Signal peptide for insertion into endoplasmic reticulum, CRD = cysteine‐rich domain (the Norrin ligand‐binding domain), TM = single pass of the transmembrane domain, CD = cytoplasmic domain, frameshifted seq = protein sequence after the frameshift due to insertion. F, Imaging of dissected retinal vasculature of 6‐month old wild‐type control (Tg[fli1:EGFP]AB) and fzd4 ins10/ins10 mutant fli1:EGFP transgenic zebrafish. Note the disorganized vasculature in the mutant. G. Measurement of vessel density shows an increase in the vascular network of fzd4 mutant fish compared to its control counterpart PHENOTYPE:
|
|
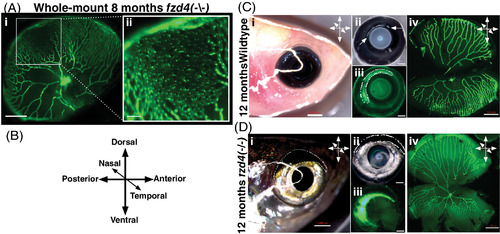
Asymmetric retinal vasculature distribution and ventral restriction of the fzd4 vascular phenotype. A, Whole mount retina from an 8‐month old fzd4 mutant. GFP marks endothelial cells (fli:eGFP). Note abnormally high vessel density and apparent fusion of nearby vessels to form disorganized endothelial sheets. Scale bar 500 μm and inset 100 μm. B, Orientation of the eyes with respect to the body axes of the fish in C, D. C‐D, Orientation of a right eye from control (C) and fzd4 (D) zebrafish using external morphology before enucleation (i), after enucleation with brightfield in C‐ii, (arrowheads depicts a bright band on the dorsal side above the lens in casper fish and in D‐ii, (dotted circle highlights a dark dorsal band in fzd4 fish, and with fluorescence in C‐iii and D‐iii (dotted circles depict a bright fluorescent band on the dorsal posterior surface in C‐iii, and D‐iii), and during dissection of the tissue (C‐iv and D‐iv). Scale bar in Ci and Di is 1 mm and in Cii‐iv, Dii‐iv is 500 μm PHENOTYPE:
|
|
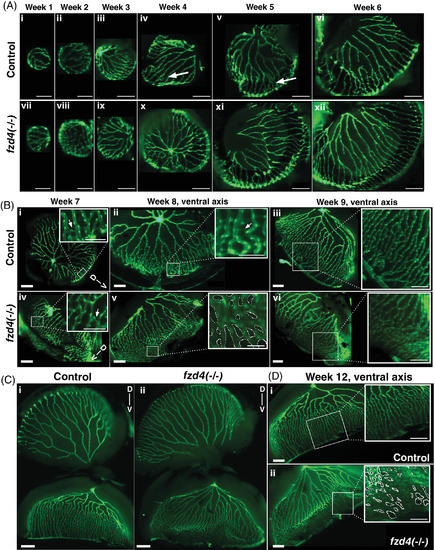
Developmental progression of zebrafish retinal vasculature in control and fzd4 fish. A, Weeks 1‐6. The retinal vasculature engulfs the entire lens as early as week 1. A few anastomoses are detected as early as week 4 in both control (arrows in iv, v) and fzd4 mutants (not shown). B, Weeks 7‐9. Ventral asymmetry can now be detected in both control and fzd4 mutants, in which the vessels on the ventral side are more closely opposed compared to those on the dorsal side. At week 8, ventral fusion is more pronounced in fzd4 mutants than in controls. Arrows in B‐i and B‐iv insets indicate sprouts. C‐D, Week 12. In, C, control and mutant retinas are shown whole to show the difference in dorsal to ventral arrangement of their vessels. In, D, only the ventral side from, C, is shown to emphasize the difference in vessel morphology and fusion between control and mutant eyes (inset shows severe fusion in a lateral section of the eye). Scale bars in, A, i‐ii, and vii‐viii is 100 μm, and A, ix‐xii is 200 μm. Scale bars in, B, i‐vi is 200 μm and in insets 100 μm. Scale bar in, C, is 500 μm. Scale bars in, D, are 200 μm, inset are 100 μm. Dotted circles in inset in, B‐v and D‐ii, highlight the spaces between blood vessels to emphasize the degree of abnormal anastomoses PHENOTYPE:
|
|
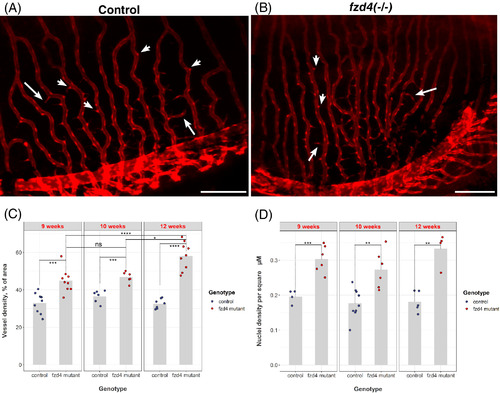
Loss of fzd4 results in increased retinal vessel density and endothelial cell proliferation. A‐B, Thin cellular projections (arrows) emanating from the red foci of the endothelial cell bodies (arrowheads) to form a kinked and branching pattern in the vasculature are detected in both, A, control and B, fzd4 mutant retinas. Green fluorescent protein (GFP) (red) marks retinal vasculature. Scale = 200 μm. C‐D, Quantification of cellular fusion and proliferation shows a progressive increase over time in vessel density of fzd4 mutants compared to controls and itself at earlier time points (compare fzd4 week 9 to fzd4 week 12). Furthermore, this increase in vessels density results from increase in endothelial proliferation as shown in D PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
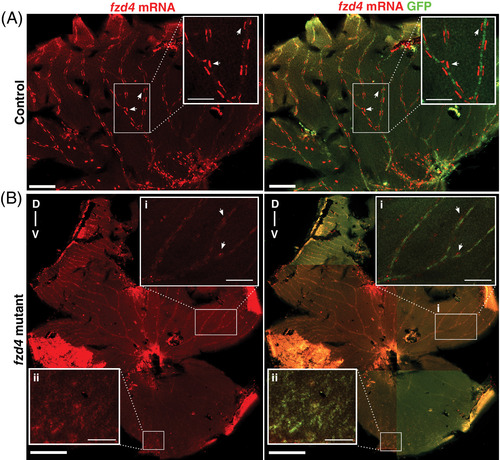
fzd4 expression is reduced in the adult retinal vasculature of fzd4 mutants. Fluorescence detection of fzd4 mRNA (red) in, A, control and B, fzd4 mutant retinal vasculature. A, In control fish, high fzd4 mRNA levels are detected in discrete regions surrounding the outer border of the blood vessel (inset). Arrowheads point at high levels of fzd4 signal in these discrete regions. Scale bar is 500 μm, inset is 100 μm. B, fzd4 mRNA (red) is reduced in fzd4 mutant fish. This reduction correlates with increased severity of vessel fusion. In the dorsal axis, reduced levels of fzd4 expression are observed (inset B‐i), while no fzd4 expression is detected in the ventral axis (inset B‐ii). Scale bar is 500 μm, inset is 100 μm. Merged panel shows immunofluorescence staining of green fluorescent protein (GFP) (green), which allows for the identification of endothelial cells and the orientation of dissected intraocular sections of the eye. The dorsal/ventral axis is denoted as D‐v |
|
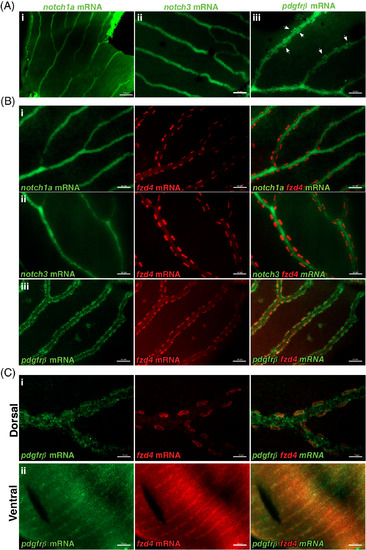
fzd4 expression is detected in pericytes. Fluorescence detection of notch1a, pdgfrß, and notch3 (green) and fzd4 mRNA (red) in, A‐B, control and C, fzd4 mutant retinal vasculature. A, In control fish, strong localization of i) notch1a and ii) notch3 expression is detected throughout the retinal vasculature but not in pericytes. Similarly, in A‐iii, strong pdgfrß expression is detected throughout the retinal vasculature, with lower levels of pdgfrß expression along and within pericytes, which are localized at the outer border of the blood vessel (arrowheads). B, Double‐RNA staining of i, notch1a, ii, notch3, and iii, pdgfrß mRNA (green) with fzd4 mRNA (red), show co‐localization of fzd4 only with pdgfrß mRNA in the pericytes. C, Co‐localization of fzd4 with pdgfrß mRNA in the pericytes is only detected on the i, dorsal but not on the ii, ventral side of fzd4 mutant retinas. Scale bar in, A‐i, is 100 μm, and in, A‐ii and A‐iii, is 50 μm Scale bar in, B, i‐iii is 50 μm. Scale bar in, C‐i, is 20 μm and in, C‐ii, is 50 μm EXPRESSION / LABELING:
|