- Title
-
Thyroid hormone regulates distinct paths to maturation in pigment cell lineages
- Authors
- Saunders, L.M., Mishra, A.K., Aman, A.J., Lewis, V.M., Toomey, M.B., Packer, J.S., Qiu, X., McFaline-Figueroa, J.L., Corbo, J.C., Trapnell, C., Parichy, D.M.
- Source
- Full text @ Elife

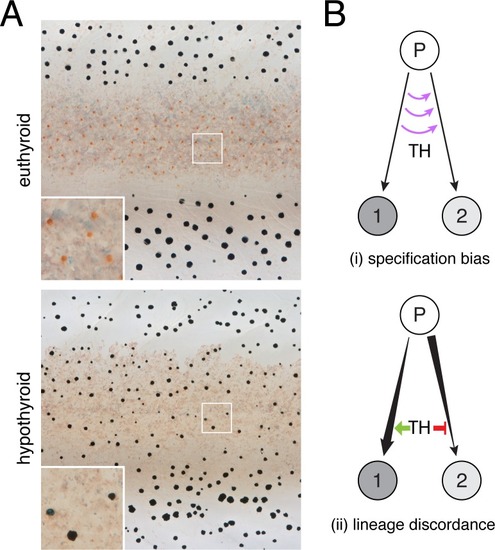
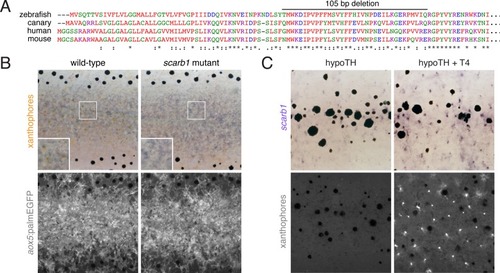
ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
( PHENOTYPE:
|
|
( |
|
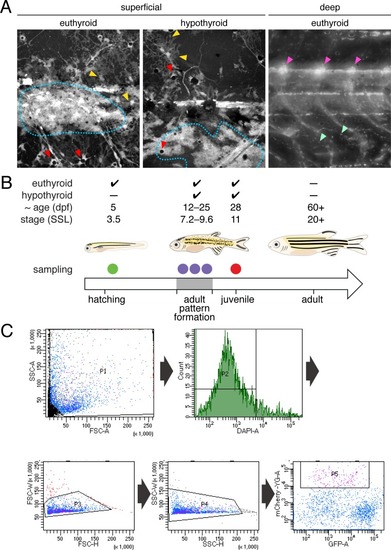
( EXPRESSION / LABELING:
|
|
( EXPRESSION / LABELING:
|
|
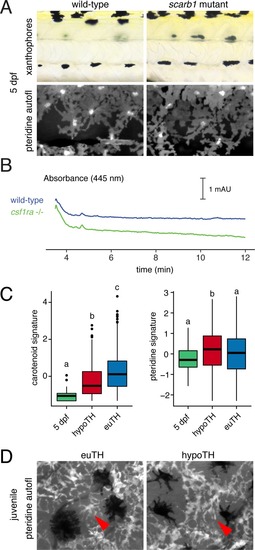
PHENOTYPE:
|
|
PHENOTYPE:
|
|
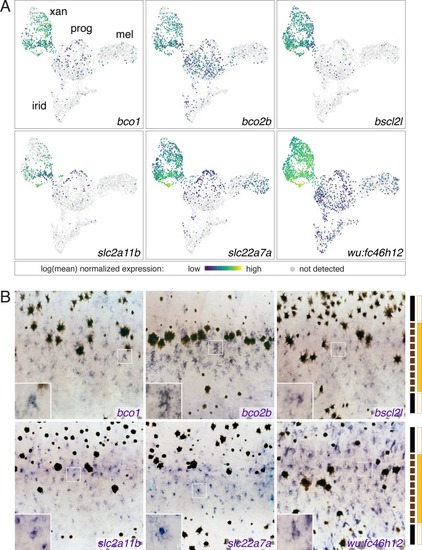
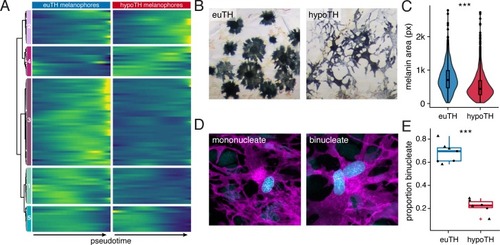
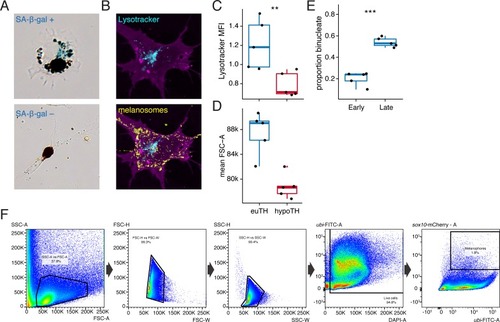
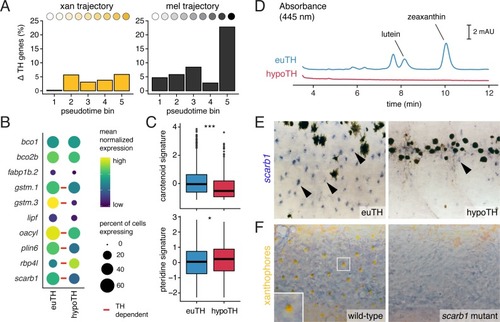
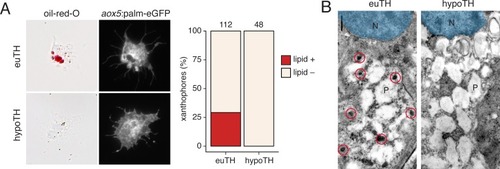
UMAP plots of pigment cell clusters colored by expression of TH-dependent genes in xanthophores: EXPRESSION / LABELING:
PHENOTYPE:
|
|
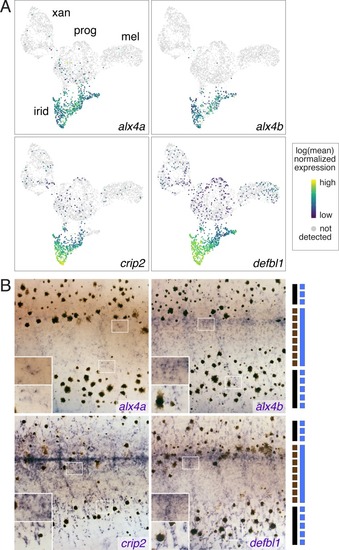
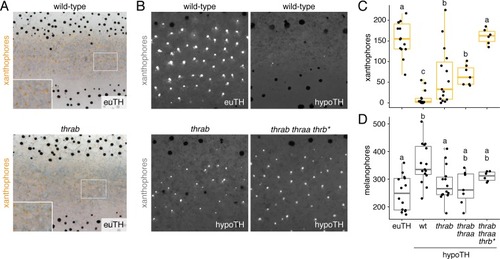
( PHENOTYPE:
|
|
( EXPRESSION / LABELING:
PHENOTYPE:
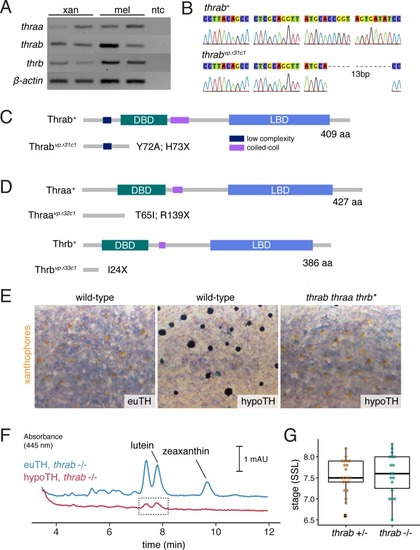
|
|
( |
|
PHENOTYPE:
|
|
( EXPRESSION / LABELING:
PHENOTYPE:
|