- Title
-
Regulation of locomotor speed and selection of active sets of neurons by V1 neurons
- Authors
- Kimura, Y., Higashijima, S.I.
- Source
- Full text @ Nat. Commun.
|
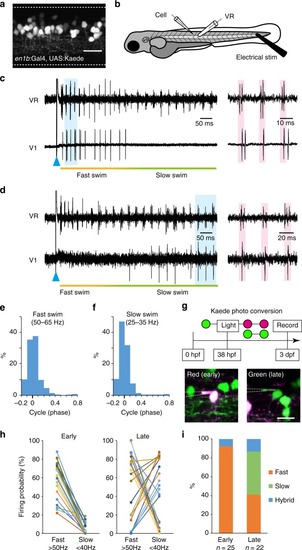
Firing patterns of V1 neurons in larval zebrafish. |
|
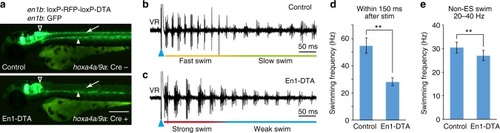
V1 ablation reduced cycle frequency in swimming. |
|
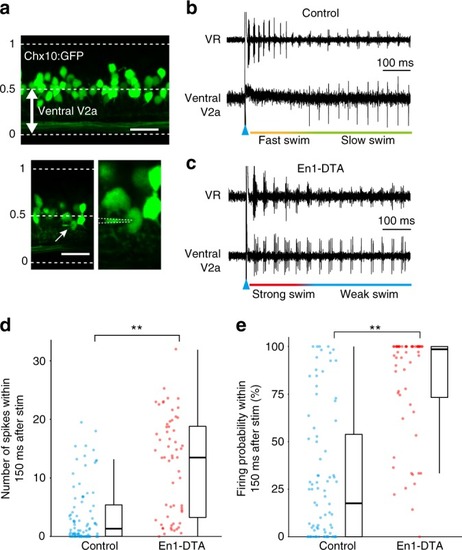
Activity of ventrally located V2a neurons in control and En1-DTA fish. |
|
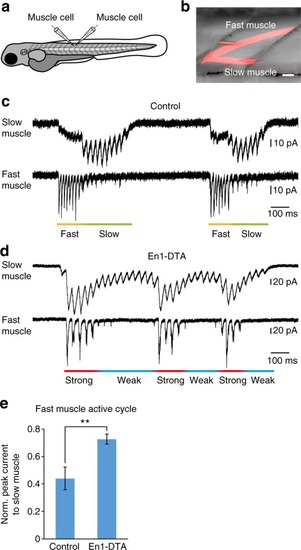
Voltage-clamp recordings of fast and slow muscles in control and En1-DTA fish. |
|
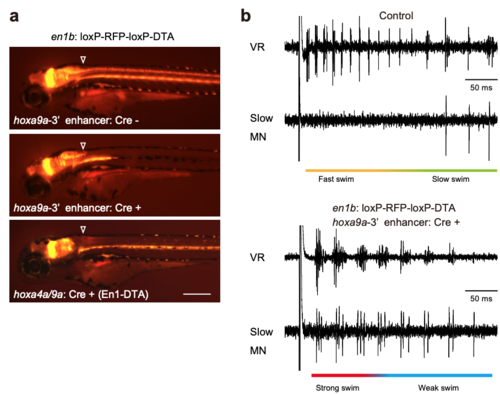
Phenotype of the compound transgenic fish of Tg [en1b:loxP-RFP-loxP-DTA] and Tg[hoxa9a-3'enhancer:Cre] (related to Figures 2 and 3) a, Fluorescent images of Tg[en1b:loxP-RFP-loxP-DTA] fish without a Cre driver (top panel), with Tg [hoxa9a-3'enhancer:Cre] (middle panel), and Tg[hoxa4a/9a:Cre] (bottom panel). Arrowheads indicate the boundary between the hindbrain and the spinal cord. Scale bar, 250 μm. b, VR and slow-type MN recordings during fictive swimming elicited by electrical stimulation in a control (top panel) and a compound transgenic fish of Tg[en1b:loxP-RFP-loxP-DTA] and Tg[hoxa9a-3'enhancer:Cre] (bottom panel). |