- Title
-
Scleraxis genes are required for normal musculoskeletal development and for rib growth and mineralization in zebrafish
- Authors
- Kague, E., Hughes, S.M., A Lawrence, E., Cross, S., Martin-Silverstone, E., Hammond, C.L., Hinits, Y.
- Source
- Full text @ FASEB J.
|
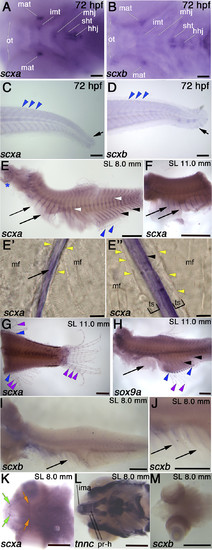
Comparison of scxa and scxb expression during embryonic and juvenile stages. In situ hybridization for indicated genes shown in lateral (C–J) or ventral view (A, B, K–M). A, B) At 72 hpf, scxa is detected in Meckel’s adductor tendon (mat), the intermandibularis tendon (imt), the mandibulohyoid junction (mhj), the sternohyoideus tendon (sht), and hyohyoideus junction (hhj). scxb is expressed at the sht and hhj, the mat, and the ocular muscle tendons (ots). C, D) At 72 hpf, scxa and scxb are expressed weakly at the myosepta (blue arrowheads) and the caudal fin (black arrows). E–J) In situhybridization for scxa (E–G), sox9a (H), and scxb (I, J) at juvenile stages as indicated, showing expression in the trunk in the posterior vertical myosepta (arrowheads), and the more anterior myosepta near the thoracic ribs (arrows), intermuscular bones (white arrowheads), fin radials (blue arrowheads), in gills (blue asterisk), and between fin bony ray segments (purple arrowheads). Sagittal sections in rib area (E′) and myosepta in anal fin area (E″) show scxa staining in intramuscular tendons separated from muscle fiber (mf) ends (yellow arrowheads) by an unstained tendon-sheath or ECM (brackets). K–M) scxa expression at juvenile stages in head tendons, including protractor hyoideus tendons (orange arrow) and intermandibularis anterior tendons (green arrows), at the attachments of the cranial muscles (labeled with tnnc) (L): Protractor hyoideus-dorsal and ventral (pr-h) and the intermandibularis anterior (ima). No scxb expression is detected in cranial tendons at juvenile stages. Scale bars, 100 µm (A–D, E′, E″), 0.5 mm (E–M).
EXPRESSION / LABELING:
|
|
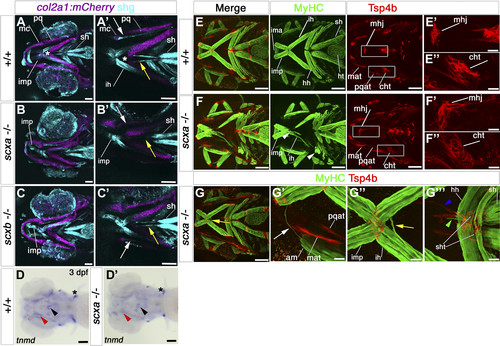
Cranial tendons, ligaments, and muscles of scxa−/− mutants are abnormal and disorganized. A–C) SHG imaging shows both collagen arrangement in tendons and ligaments and myosin heads in muscle (light blue) combined with confocal imaging of the transgene TgBAC(col2a1a:mCherry)hu5910 (cartilage, purple) of 7 dpf scxa−/−, scxb−/−, and wild-type (+/+) larvae. Shown are representative maximum projection images of the stacks (A–C) and single scans at comparable Z positions (A′–C′). Decreased SHG signal was observed in the ligaments connecting the Meckel’s and palatoquadrate cartilages of scxa and scxb mutants (white arrows), and in the sternohyoideus tendon (yellow arrows) connecting the sternohyoideus muscle and the basihyal cartilage (asterisk) of scxa mutants. D) In situ hybridization for tnmd for 3 dpf scxa−/− and their siblings (+/+) showing reduced tnmd mRNA levels in scxa mutant, such as the sternohyoideus tendon (black arrowhead) and the ligaments connecting the Meckel’s and palatoquadrate cartilages (red arrowhead). Expression at the base of the cleithrum is maintained (asterisk). E–G) Confocal stacks of 4 dpf embryos from a scxa+/− incross, immunostained for MyHC (A4.1025, green) and Tsp4b (red). Specific defects in tendon structure and directionality in scxa mutants (white rectangles) (E) are shown in higher magnification panels for mandibulohyoid junction (E′, F′) and ceratohyal tendon (E″, F″). Scxa mutants show varied array of muscle defects, such as abnormal overextension or crossing the midline of muscle fibers (F, G compared with E) and detached fibers in interhyoides and hyohyoides (arrowheads, F). The magnified area (G′–G″′) shows ectopic fiber in the adductor mandibularis (white arrow, G′), ectopic misguided fiber from the interhyoides is crossing the midline and growing toward the interhyoides across the midline (yellow arrow, G″), and ectopic extention of sternohyoides tendon (blue arrowhead) is attracting overextended fibers (green arrowhead). Am, adductor mandibularis; cht, ceratohyal tendon; hh, hyohyoides; ih, interhyoides; ima, intermandibularis anterior; imp, intermandibularis posterior; mat, Meckel’s adductor tendon; mc, Meckel’s cartilage; mhj, mandibulohyoid junction; pq, palatoquadrate cartilage; pqat, palatoquadrate adductor tendon; sh, sternohyoideus; sht, sternohyoides tendon. All images in ventral view, anterior to left. Scale bars, 100 µm, except F′–F″ and G′–G″′, 20 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
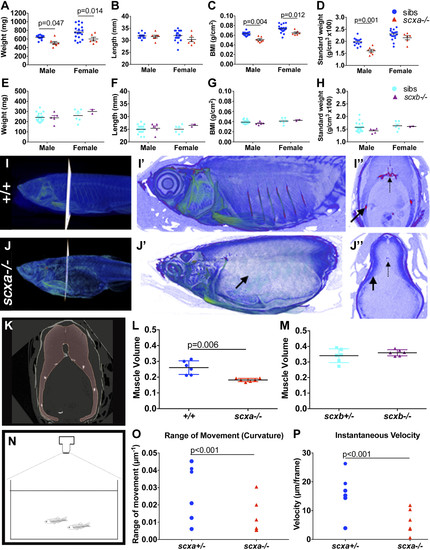
Lack of Scxa in adults affects body measurements and swim behavior. A–H) Weight, length, BMI, and standard weight comparison between mutants and sibling adult fish from a scxa+/− incross (13 siblings and 7 scxa−/− males, 18 siblings and 6 scxa−/− females, A–D) and a scxb+/− incross (21 siblings and 5 scxb−/− males, 6 siblings and 2 scxb−/− females, E–H). Males and females are presented separately as were found significantly different. I, J) Contrast-enhanced µCTs to show soft tissue of scxa−/− mutant (J–J″) and a +/+ sibling (I–I″). Magnified scans show lack of ribs (black arrows) in the mutant. I″, J″) Transverse optical sections at indicated positions, where dashed arrows indicate the vertebrae. K) Myotome volume from co-reared similar-length fish are calculated from scans by creating a virtual steak between 2 ribs (red area, see Materials and Methods). L, M) Quantification of muscle volume in scxa−/− adult mutants (L) and scxb−/− (M) and their respective siblings. N–P) Swimming performance was calculated from videos taken from above the tank [schematic drawing in panel (N), see also Supplemental Video and Supplemental Fig. S7], and the range of movement (curvature, M) and instantaneous velocity (N) were calculated as detailed in the Materials and Methods. Two-way ANOVA statistics with Sidak’s post hoc tests were performed (A–H). Unpaired t test with Welch’s correction (L, M) and 2-way ANOVA (O, P). Significant P-values indicated on graphs. PHENOTYPE:
|
|
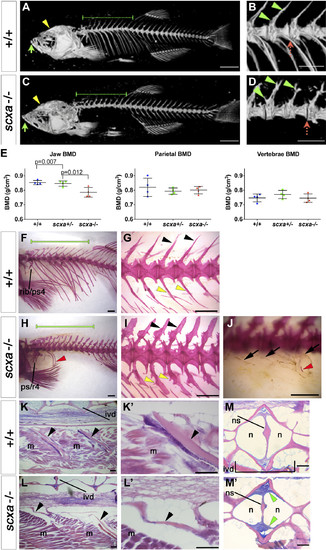
Adult scxa homozygous mutants have skeletal abnormalities. A–E) Three-dimensional volumetric isorenders from µCt data of scxa−/− and wild-type sibling showed absence of mineralized ribs (green dashed line above the rib region) (C, compared to A), and protruding jaws (green arrows) in mutants. Thoracic ribs region was magnified to show details of the vertebrae (B, D). Small bony structures were observed branching from the haemal arches (green arrowheads), and vertebrae misalignments were often present (red dashed arrow) in scxa mutants. E) BMD values were calculated from 3 distinctive bones: jaw (green arrows in A, C), vertebrae, and the parietal bone (yellow arrowhead in A, C). One-way ANOVA statistics with Tukey’s post hoc test performed, P values are as indicated. F–J) AR-stained adult scxa−/− mutants show no signal in ribs (H, under green line, compared with F), and neural (black arrowheads) and haemal arches (yellow arrowheads) have extensive bony growth (vertebrae 15–19 magnified in G, I). Fibrous, almost transparent ribs are seen in high magnification (black arrows, J), with the odd mineralized rib tissue (red arrowhead in H, J). The area rostrally to rib 5 is formed normally. Ps/r4, parapophysis and rib 4. K–M) Adult wild-type and scxa mutant zebrafish sagittal (KK′,LL′) and transversal (MM′) paraffin sections stained with H&E and AB. Existing rib structure in scxa mutant is short, thin, and wavy. The IVD is shown in MM′. The notochord string (ns) connecting the dorsal and ventral of the V shape is normal. However, the IVD is enriched with fibrous tissue (more purple, green arrowheads). M, muscle; n, notochordal cells. Scale bars, 1 mm (A–D, F–J), and 100 µm (K–M). |
|
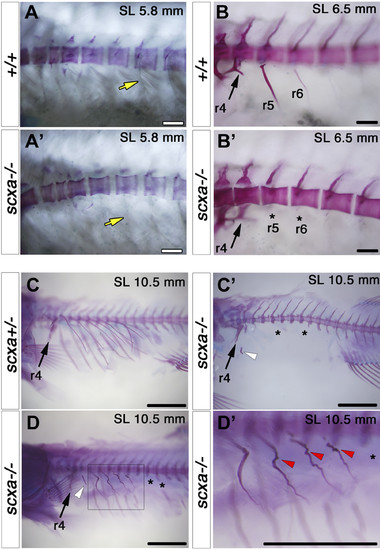
Scxa is is required for rib mineralization and its patterning. AR staining for fish from a scxa+/− incross at the indicated juvenile stages. A) At SL 5.8 mm, increased differential interference contrast (DIC) shows that the junction region between muscle and rib (yellow arrows) is already abnormal (AA′). B) At SL 6.5 mm, the first anterior ribs (r5 and r6) are lacking (asterisks in B′, compared with B). C) A severe lack of mineralized ribs (black asterisks) and mineralized rib fragments (white arrowhead) is seen at SL 10.5 mm (C′, compared with C). D) In another mutant (D), some ribs are missing (asterisks in D), whereas others are highly fractured and healed (r5–r9 magnified and marked by red arrowheads in D′). Note that r4 is formed normally (arrows in B–D). All scale bars, 100 µm. PHENOTYPE:
|
|
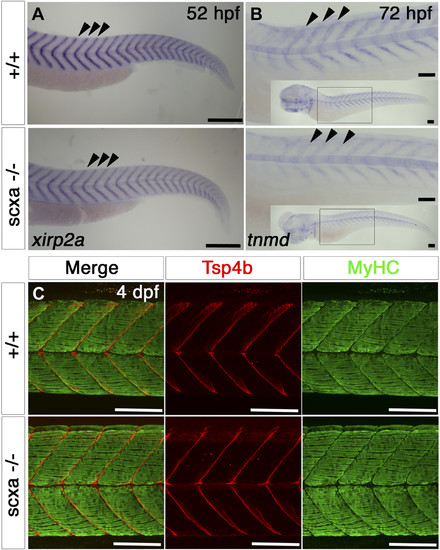
Scxa mutants have reduced levels of tendon markers, but somitic MTJs appear normal. A, B) In situ hybridization for xirp2a at 52 hpf (A) and tnmd at 3 dpf (B) for scxa−/− and their siblings (+/+), lateral view, anterior to left. B) The main image shows the anterior somites, inset-whole embryo. Both xirp2a and tnmd mRNA levels are reduced at the MTJs at the somitic borders (arrowheads). C) Confocal stacks of immunodetection of MyHC (A4.1025) and Tsp4b in somites 11–14 of 4 dpf embryos of scxa−/− and siblings showing normal distribution of Tsp4b and normal muscle structure. All scale bars, 100 µm. |
|
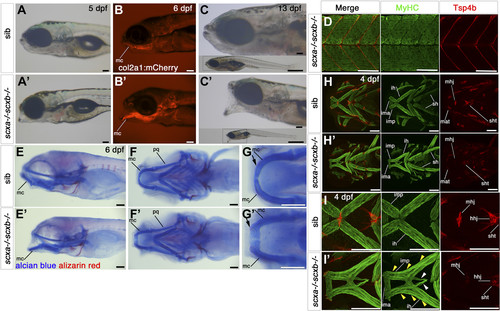
Scxa;scxb double mutants have a jaw phenotype and severe musculoskeletal defects. A–C′) Live transmitted light and red fluorescent images in lateral view, anterior to left of genotyped scxa−/−;scxb−/− double mutants (A′–C′) compared with siblings (A–C, shown is scxa+/−scxb+/−). Mutants show a hanging open jaw. The col2a1:mCherrytransgene (B) shows the dropping Meckel’s cartilage (mc). At 13 dpf, fish are much smaller than siblings (whole-fish insets in C). D) Confocal stacks of immunodetection of MyHC (A4.1025) and Tsp4b in 4 dpf embryos of scxa−/− scxb−/− showing normal distribution of Tsp4b and normal muscle structure (compare with Fig. 6C). E–G′) AB/AR staining for cartilage and bone for scxa−/−scxb−/− (E′–G′) and sibling from the same cross (E–G) shown in lateral (E) and ventral (F, G) views that show the hanging jaw phenotype. The magnified area from mc shows many less differentiated rounded cells at its most anterior tip, near the joint (arrow, G′) compared with elongated mature cells in sibling (arrow, G). H–I′) Confocal stacks showing immunodetection of cranial muscles (MyHC, A4.1025) and tendons (Tsp4b) of 4 dpf embryos of scxa+/−; scxb+/− incross in ventral view. Tsp4b is highly reduced in tendons and ligaments of double mutants as shown for mandibulohyoid junction (mhj) (magnified area, II′). Many muscle fibers in scxa−/−; scxb−/− extend the length of the intermandibularis posterior (imp) and the interhyoideus (ih) (yellow arrowheads, I′), whereas others extend far beyond their normal end at the mhj until their meeting point (white arrowheads, I′). All scale bars, 100 µm. Hhj, hyohyoideus junction; ih, interhyoides; ima, intermandibularis anterior; imt, intermandibularis tendon; mat, Meckel’s adductor tendon; mc, Meckel’s cartilage; pq, palatoquadrate cartilage; sh, sternohyoideus; sht, sternohyoideus tendon.
PHENOTYPE:
|
|
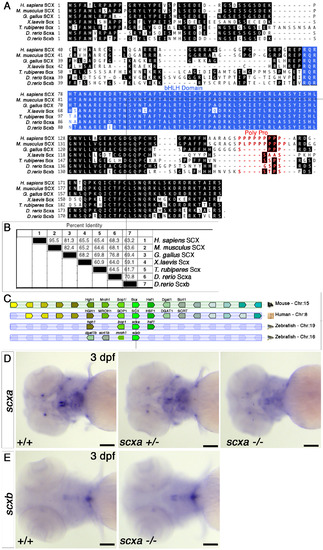
Scxa is the more conserved homologue of the mammalian Scx. A. Clustal alignment of the translation product of the zebrafish scxa and scxb genes with representatives of other major vertebrate groups (human, mouse, chicken, Xenopus laevis and Fugu rubripes). B. Sequence pair distances of representative the above proteins using the Clustal method with PAM 250 residue weight table. C. Synteny diagram based on Genomicus software and Ensembl GRCz11 showing similar position of scxa gene inside intron 3 of the Bop1 gene on the other strand, as described for mouse Scx (11), whereas scxb locus show changes and rearrangements compared with mouse and human genomes. D-E. In situ hybridisation for scxa (D) and scxb (E) for 3 dpf embryos from a scxa+/- incross. Cranial expression is shown in ventral view. scxa mRNA levels in scxa mutant (11/50 embryos from the incross) are reduced compared with heterozygoute (29/50) and +/+ embryos (10/50), genotypes confirmed (D). scxb mRNA levels and spatial pattern do not differ between genotypes, genotypes confirmed (E). scale bars-100μM. |
|
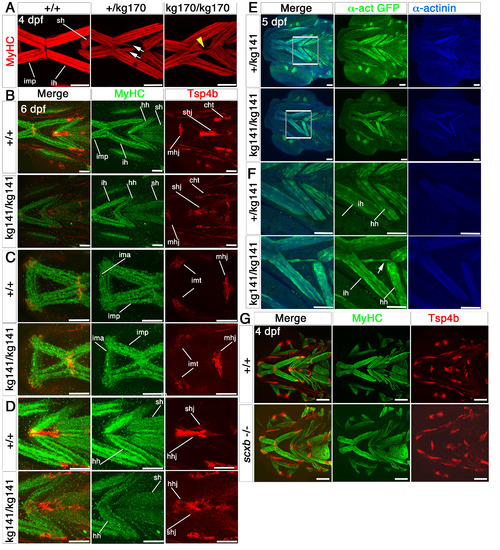
Cranial tendons, ligaments and muscles of scxakg141 mutants are abnormal and disorganized. Confocal stacks of cranial muscles using immunofluorescence for MyHC (A4.1025, red in A), Tsp4b (red) and MyHC (MF20, green) in B-D, GFP (green) and alpha-actinin (blue) in E,F and Tsp4b (red) and MyHC (A4.1025, green) in G. All in ventral view, anterior to left. A. Embryos from a scxakg170/+ incross at 4 dpf; scxa-/- mutants had misaligned fibres and tri- and four-way abnormal junctions (yellow arrowhead). Some scxa+/- embryos had some milder defects (white arrows). B-D. 6 dpf embryos from a scxakg141/+ incross; the matrix protein, Tsp4b was downregulated in mutants, and some tendons such as the mandibulohyoid junction and intermandibular tendon (C), the sternohyideus and hyohyoideus tendons (D) were misshapen and showed decreased matrix condensation. Muscle fibre defects contained fibres connecting to wrong muscles and disorganized junctions. E,F. 5 dpf embryos from a scxakg141/+;Tg(actc1b:egfp)zf13 incross. F shows magnified boxed region in E. Mutant embryos had fibres from the interhyoideus muscle growing in the wrong direction towards the hyohyoideus junction (white arrow). G. Both tendons and muscles of 4 dpf embryos from a scxb+/- incross looked normal. mhj, mandibulohyoid junction, ima, intermandibularis anterior, imp, intermandibularis posterior, imt, intermandibular tendon, sht, sternohyoides tendon, hhj, hyohyoideus junction, ih, interhyoideus, hh, hyohyal, sh, sternohyoides. All scales 100μm except A, 50μm. |
|
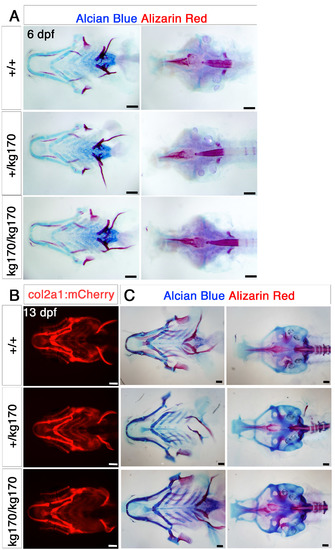
Cranial bone and cartilage show no obvious defects at embryonic and juvenile stages. A. Alcian blue and Alizarin red double staining for cartilage and bone for 6 dpf scxa+/kg170 incross embryos, showing as flatmounts of the pharyngeal skeleton (ventral view, left) or neurocranium (dorsal view, right). B-C. 13 dpf scxa+/kg170;Tg(col2a1:mCherry) incross juveniles shown for live mCherry expression (ventral view, B) and Alcian blue and Alizarin red double staining (pharyngeal skeleton, ventral view, C, left and neurocranium, dorsal view, C, right). No dramatic differences are detected beyond normal variation. |
|
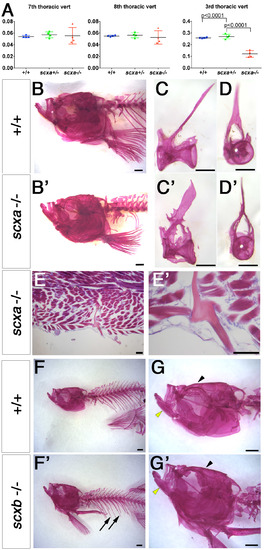
Adult scxa mutants show skeletal defects in trunk but not in skull. A. Vertebral centrum volumes calculated from μCT scans of genotyped adults from a scxa+/- incross (n=3 per genotype) using the minimum volume possible around the neural canal, excluding (for 7th and 8th thoracic vertebrae) or including (for the 3rd thoracic vertebra) all processes, trabeculae, spines and ribs in a transverse view. Vertebrae centrum alone is similar between all genotypes, but when the above skeletal elements were added, volume in scxa-/- fish is significantly smaller than siblings. One-way ANOVA statistics with Tukey’s post-hoc test performed, p-values indicated. B-D’, F-G’. Alizarin Red staining for adult scxa-/- (B’-D’) and siblings (B-D) or scxb-/- mutants (F’,G’) and their siblings (F,G). Skull of scxa mutants appeared normal whereas staining in ribs was missing. Skull (arrowhead), jaw (yellow arrowhead) and ribs (arrows) looked normal in scxb mutants. Dissected thoracic 12th vertebrae from scxa-/- mutant and sibling are shown in lateral and frontal views for details of bony growth in arches (C’,D’ compared with C,D). E, E’. Adult scxa mutant zebrafish sagittal paraffin section stained with Hematoxylin and Eosin and Alcian blue showing fractured and healed rib fragment (magnified in E’). Scale bars, 100μm in E,E’, 1mm in B,B’,F,F’, 0.5mm in C-D’. |
|
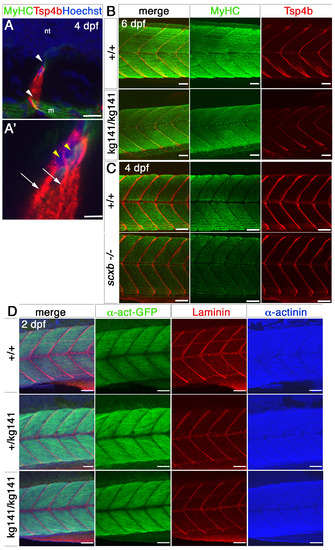
Myotendinous junctions and muscle of scxakg141 and scxb mutants appear normal. A-A’. Small confocal stacks of immunofluorescence for MyHC (A4.1025, green), Tsp4b (red) and nuclear stain (Hoechst) of 4 dpf wt embryos somites 11,12, in lateral view, showing cells (magnified in A’) at the edge of myosepta (white arrowheads) surrounded by the somites and the neural tube (nt), in which single nuclei (yellow arrowheads) are surrounded by Tsp4b matrix stain and extend processes of Tsp4b along the myosepta (arrows). m, muscle fibres. B,C. Lateral view of somites 11- 14 of confocal stacks for immunofluorescence of MyHC (A4.1025, green), Tsp4b (red) of 6 dpf embryos from a scxakg141/+ incross (B) or 4 dpf of a scxb+/- incross (C). No major differences in either MyHC or Tsp4b are detected. D. Lateral view of confocal stacks for GFP, Laminin and sarcomeric alpha-actinin of somites 11-14 from 2 dpf scxakg141/+;Tg(actc1b:egfp)zf13 incross embryos. No major differences between genotypes are detected. All scale bars 50μm, except A-A’, 20μm. |