- Title
-
Morphogenesis and axis specification occur in parallel during optic cup and optic fissure formation, differentially modulated by BMP and Wnt
- Authors
- Eckert, P., Knickmeyer, M.D., Schütz, L., Wittbrodt, J., Heermann, S.
- Source
- Full text @ Open Biol.
|
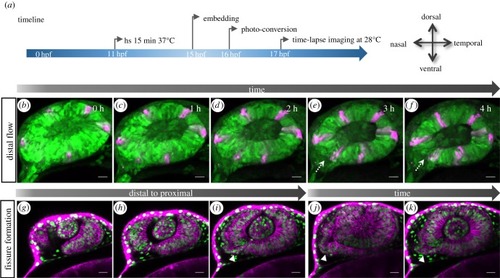
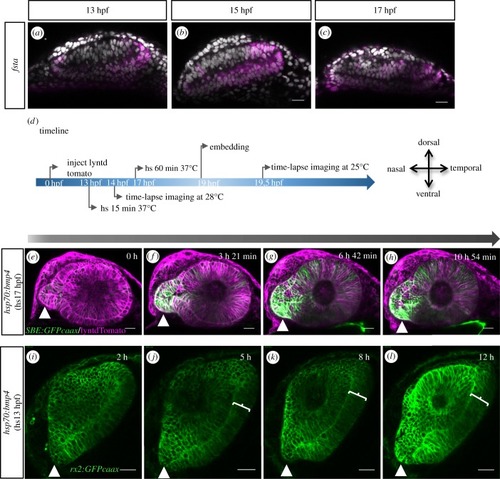
Transformation of the optic vesicle into the optic cup. ( |
|
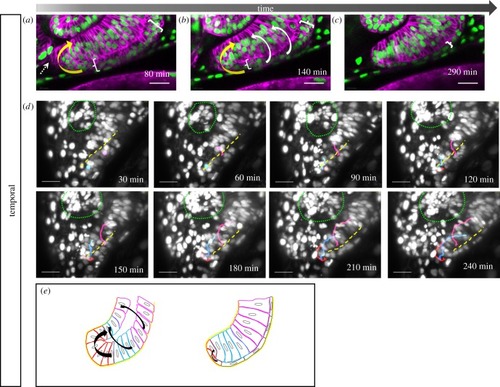
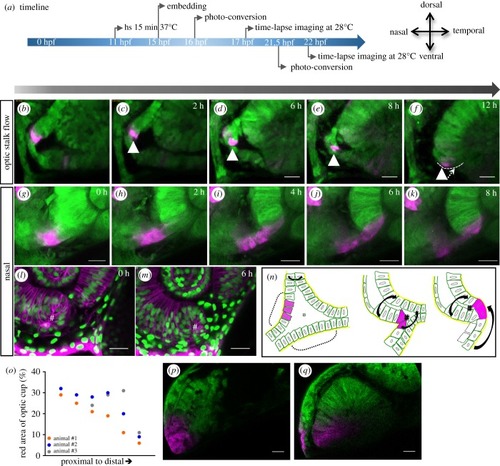
Development of the temporal fissure margin. ( |
|
Development of the nasal fissure margin. ( |
|
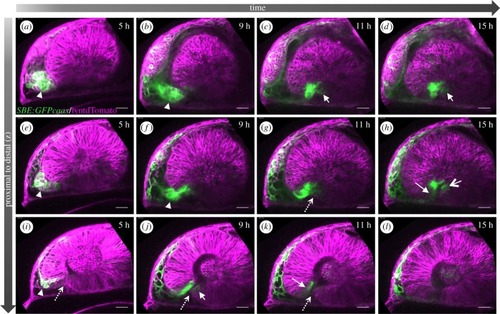
TGFβ-signalling-positive cells are secondarily added to the optic fissure margins. ( |
|
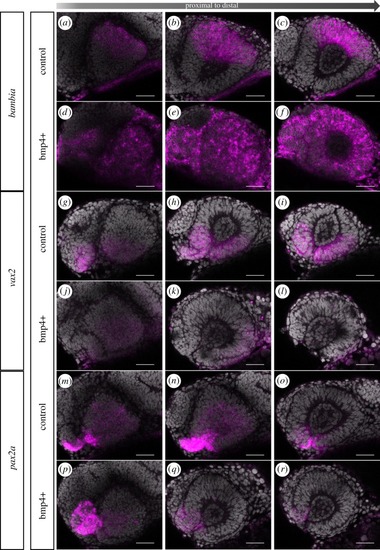
Induced expression of bmp4 hampers optic fissure formation. |
|
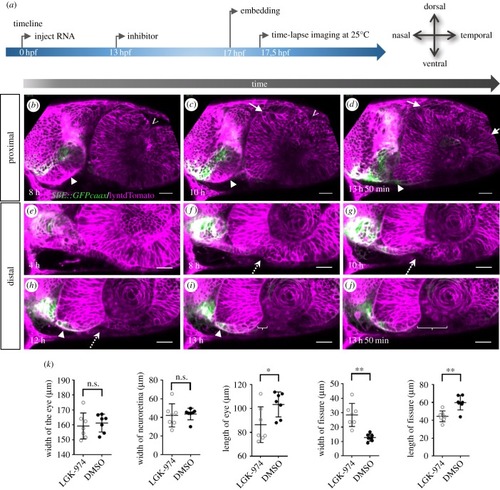
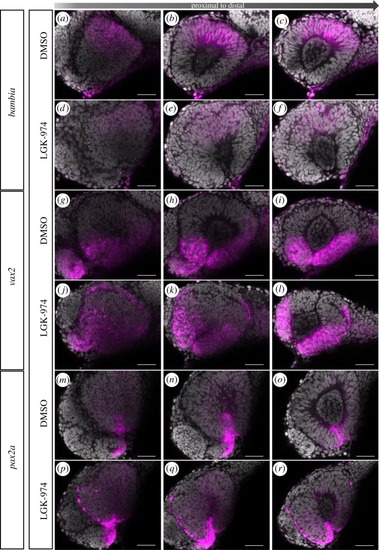
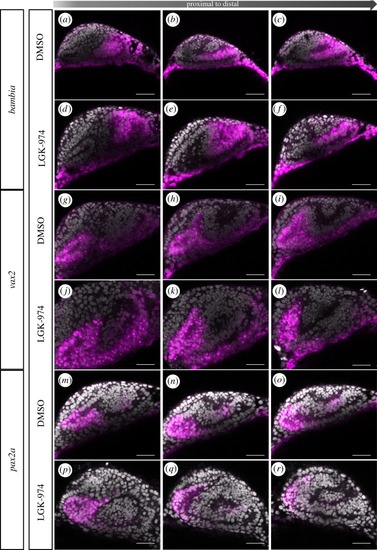
Wnt-signalling inhibition affects optic cup morphogenesis and prevents TGFβ-signalling positive cells from entering the ventral part of the optic cup. ( |
|
Induced bmp4 expression affects moprphogenesis and axis specification. |
|
Inhibition of Wnt-signalling affects morphogenesis. |
|
Inhibition of Wnt-signalling does not affect dorsal ventral axis specification. |