- Title
-
Clec14a genetically interacts with Etv2 and Vegf signaling during vasculogenesis and angiogenesis in zebrafish
- Authors
- Pociute, K., Schumacher, J.A., Sumanas, S.
- Source
- Full text @ BMC Dev. Biol.
|
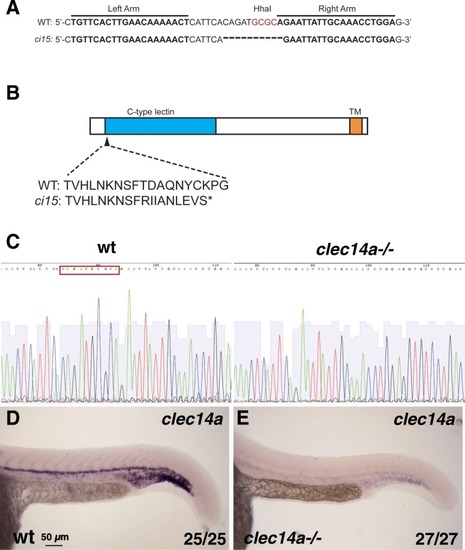
Generation of clec14a mutants by TALEN-mediated genome editing. a Wild-type and clec14aci15 mutant sequence showing the binding regions for TALE nucleases and the 10 bp deletion in the clec14a mutants which includes the deletion of HhaI restriction site used for genotyping. b A diagram of Clec14a protein sequence which includes C-type lectin and transmembrane (TM) domains. clec14aci15 mutation is predicted to cause a frameshift early in the protein coding sequence starting at the amino acid 44 and would lead to a premature stop codon. c, d DNA sequencing chromatogram shows a 10 bp deletion (boxed-in wild-type sequence) in the cDNA of clec14a mutants. d, e In situ hybridization analysis for clec14a expression in wt embryos (d) and clec14a mutants (e) at 24 hpf. Note a significant reduction of clec14a expression in clec14a mutant embryos. The experiment has been replicated twice; the combined number of embryos analyzed and showing the phenotype is shown in the lower right corner
EXPRESSION / LABELING:
PHENOTYPE:
|
|
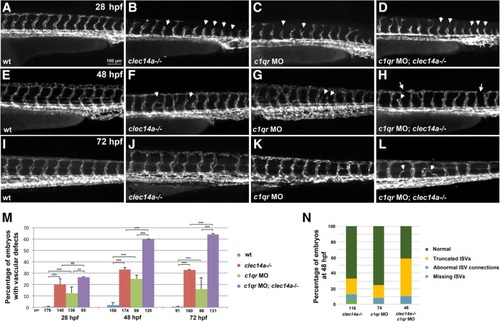
Clec14a and C1qr have a partially redundant function during angiogenic sprouting. Kdrl: GFPexpression in the trunk region imaged by fluorescent microscopy at 28 hpf (a-d), 48 hpf (e-h) and 72 hpf (i-l). a, e, i Wild-type control embryos; b, f, j clec14a−/− mutants; c, g, k Wild-type embryos injected with 10 ng of c1qr MO; d, h, l clec14a−/− mutant embryos injected with 10 ng of c1qr MO. Note the delayed intersegmental vessel sprouting in clec14a mutants (b) and c1qr MO embryos (c) at 24 hpf (arrowheads). Partial sprouts (arrowheads) and abnormal vascular connections (arrows) are apparent in the clec14a−/−; c1qr MO embryos (d, h, l). m Percentage of embryos with vascular defects. ** p < 0.01; *** p < 0.001; NS, no significance, Fisher’s exact test. Data were combined from 3 independent experiments. Error bars show standard error. n Distribution of different types of phenotypes in embryos at 48 hpf. Data were combined from two independent experiments
PHENOTYPE:
|
|
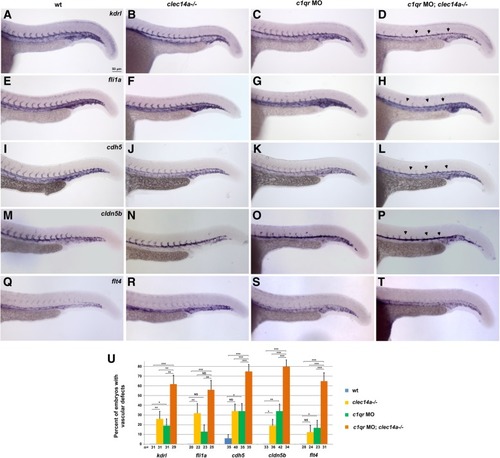
Analysis of vascular marker expression by in situ hybridization at 24 hpf in clec14a−/− embryos injected with 10 ng of c1qr MO. a-d kdrl, e-h fli1a, i-l cdh5, m-p arterial marker cldn5b, q-t venous marker flt4expression. Note the strong inhibition of angiogenic sprouting in the double clec14a−/−; c1qr MO embryos (arrowheads). Only weak or partial inhibition of sprouting is observed in clec14a−/− or c1qrMO embryos. u Percentage of embryos with defects in ISV sprouting is greatly increased among c1qrMO; clec14a−/− embryos compared to c1qr MO or clec14a−/− embryos. n refers to the number of embryos analyzed for each marker. *p < 0.05; ** p < 0.01; *** p < 0.001; NS, no significance, Fisher’s exact test. Data were combined from two independent experiments. Error bars show standard error |
|
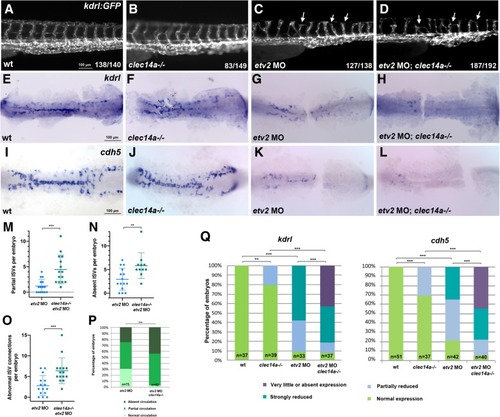
Combinatorial interaction between etv2 and clec14a. a-d ISV sprouting defects (arrowheads) are increased in clec14a mutants injected with the low dose of etv2 MO2 (0.25 ng) compared with etv2 MO injection in wild-type embryos. kdrl: GFP transgenic embryos were imaged at 48 hpf; the trunk region is shown. e-l ISH analysis for kdrl (e-h) and cdh5 expression at the 15–16-somite stages. Flat-mounted embryos; only the trunk and tail region is shown. Note a significant reduction in cdh5 and kdrlexpression in the embryos injected with low dose (0.125 ng) of etv2 MO2. Much greater reduction is observed in etv2 MO; clec14a−/− embryos. m-o The number of partial (m) or absent (n) ISVs per embryo and abnormal ISV connections per embryo (o) in wild-type kdrl: GFP or clec14a−/−; kdrl: GFP embryos at 48 hpf which were injected with 0.25 ng of etv2 MO. 12–15 embryos were analyzed for ISV defects. ***p < 0.001, t-Student’s test. p Percentage of embryos with affected blood circulation at 48 hpf. All wild-type control and clec14a−/− embryos had normal blood circulation (not shown). To calculate statistical significance, embryos were compared with normal or defective circulation (combined partial and absent circulation categories) using Fischer’s exact test, ***p < 0.001. q Percentage of embryos with normal or reduced kdrl and cdh5 expression. Statistical significance was calculated for wild-type versus clec14a−/− and etv2 MO embryos using normal and reduced expression categories (all categories with reduced expression were combined), and for etv2 MO; clec14a−/− embryos versus clec14a−/− and etv2MO embryos using very little or absent expression categories (normal, partially and strongly reduced expression categories were combined). ** p < 0.01, *** p < 0.001, Fisher’s exact test. Data were combined from two independent experiments EXPRESSION / LABELING:
PHENOTYPE:
|
|
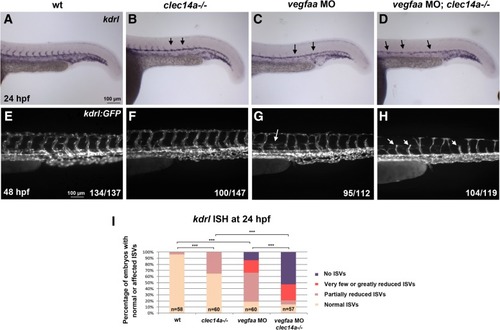
clec14a mutation and vegfaa MO knockdown show synergistic effect in inhibiting intersegmental vessel (ISV) sprouting. a-d ISH analysis of kdrl expression at 24 hpf. e-h Analysis of kdrl: GFP expression at 48 hpf. Note that partial inhibition of sprouting is observed using low dose of 2.5 ng vegfaa MO in wt embryos (c, g). The same dose in clec14a mutants results in a complete loss of sprouting at 24 hpf and significant inhibition of sprouting at 48 hpf (d, h). i Percentage of embryos with normal or inhibited ISVs based on kdrl expression at 24 hpf. *** p < 0.001, Fisher’s exact test; embryos with partially reduced ISVs were compared between wt and clec14a−/− or vegfaa MO groups; embryos with no ISVs were compared between the double knockdown vegfaa MO; clec14a−/− and the other groups. Data were combined from two independent experiments
|