- Title
-
PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo.
- Authors
- Loynes, C.A., Lee, J.A., Robertson, A.L., Steel, M.J., Ellett, F., Feng, Y., Levy, B.D., Whyte, M.K.B., Renshaw, S.A.
- Source
- Full text @ Sci Adv
|
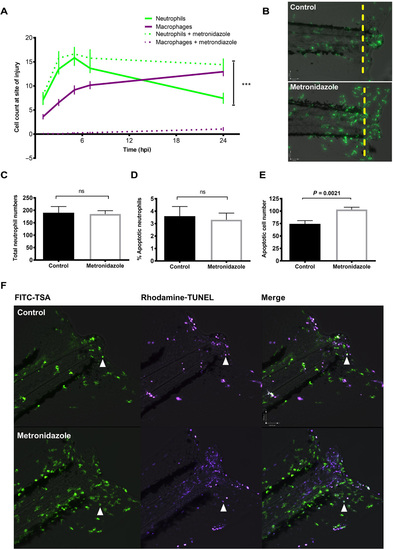
Macrophage clearance of apoptotic cells is necessary for successful resolution of neutrophilic inflammation in an in vivo zebrafish model. (A) Neutrophil and macrophage counts at the wound in triple transgenic Tg(cfms:Gal4)i186;Tg(UAS:nfsB-mCherry)i149;Tg(mpx:EGFP)i114 larvae in the presence or absence of metronidazole. Neutrophil numbers are significantly higher at the wound in the absence of macrophages at 24 hpi, ***P < 0.0001. Statistics: Two-tailed nonpaired t test comparing neutrophil counts at 24 hpi with or without macrophage present. All data are n = 18 from three individual experiments plotted as means ± SEM. (B) Representative photomicrographs at 24 hpi showing increased neutrophil numbers at the site of injury in metronidazole-treated larvae compared to control. Wound area classed as area to the right of the yellow dashed line. Images were taken using ×10 magnification on a TE2000U inverted microscope (Nikon). (C) Total neutrophil numbers are not affected in the absence of macrophages. Data are n = 17 individual larvae. (D) Dual fluorescein isothiocyanate (FITC)–TSA and Rhodamine-TUNEL–positive cell counts in 8 dpf larvae at 24 hpi in the presence or absence of metronidazole show no significant difference (ns) in the percentage of apoptotic neutrophils. (E) Total apoptotic cell counts of TUNEL-positive cells in 8 dpf larvae at 24hpi. Macrophage-depleted larvae have significantly more apoptotic bodies at the wound, P = 0.0021. All data are presented as means ± SEM, n = 12, from two individual experiments for (D) and (E). (F) Representative photomicrographs of 8 dpf larvae at 24 hpi treated with either dimethyl sulfoxide (DMSO) control or metronidazole and dual-stained with FITC-TSA to label neutrophils and Rhodamine-TUNEL to label apoptotic cells at the wound (white arrow head). Images were taken using ×10 magnification on a TE2000U inverted microscope (Nikon). |
|
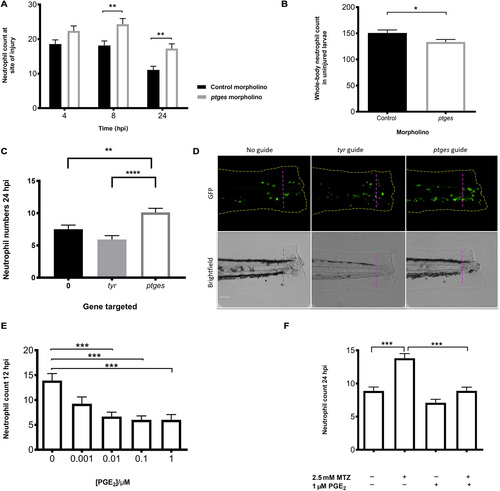
In vivo effects of PGE2 on neutrophils during an inflammatory response through genetic manipulation and PGE2 supplementation. (A) Morpholino knockdown of endogenous ptges shows the necessity of PGE2 during inflammation resolution, with a significant increase in neutrophil numbers at the wound site at 8 and 24 hpi. *P < 0.01, two-way analysis of variance (ANOVA) with Bonferonni’s posttest. Data are n = minimum 30 from three experimental repeats. (B) Total neutrophil numbers in unstimulated larvae are reduced (P < 0.05, n = 24 larvae) in the absence of ptges, indicating that the increase during an inflammatory response is not due to increased neutrophils numbers overall. (C) Recapitulation of morphant phenotype in crispant. Three days post fertilization larvae with CRISPR/Cas9-mediated knockdown of ptges display significantly more neutrophils at the wound site at 24 hpi compared to a control-injected guide group targeting tyr. Injured crispants phenocopy ptges morphants. **P < 0.01, ****P < 0.0001, one-way ANOVA with Bonferroni posttest. Data are plotted as means ± SEM with a combined minimum of 40 larvae per group from three experimental repeats. (D) Representative images of crispant larvae at 24 hpi. Area to the right of the magenta dashed line indicates the wound site where GFP neutrophils are counted. Brightfield images demonstrate successful tyr knockdown as a reduction in pigmentation is visible. (E) Dose-response showing increasing concentrations of PGE2 significantly drive neutrophilic inflammation resolution. PGE2 was added at 8 hpi with neutrophil counts performed at 12 hpi. Neutrophil numbers at the site of injury are significantly reduced between 0.01 and 1 μM. (F) In the absence of macrophages, exogenous PGE2 is able to significantly reduce neutrophil numbers back to basal levels at 24hpi. ***P < 0.001, one-way ANOVA with Bonferroni posttest. Minimum of 32 larvae from three repeats. MTZ, metronidazole. PHENOTYPE:
|
|
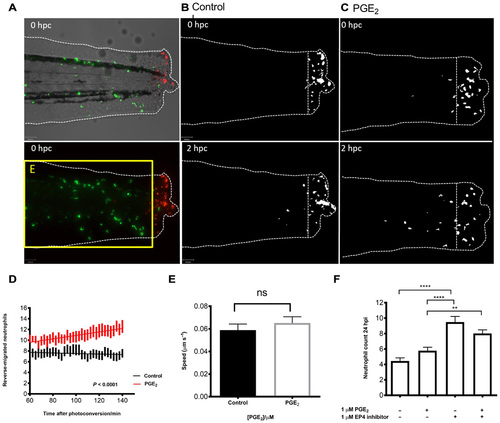
PGE2 drives accelerated reverse migration through EP4 receptor signaling. (A) Representative photomicrographs of control or PGE2-treated 3 dpf Tg(mpx:gal4)sh267;Tg(UAS:kaede)i222 larvae following photoconversion. The area to the right of the white dashed vertical line indicates wound site, where cells were photoconverted from green to red fluorescence. The yellow box E indicates the area into which the photoconverted cells migrate and corresponds to data in (D). (B) The red channel only is shown as a binary image of a control larva at 0 and 2 hours post conversion (hpc). Very little migration away from the wound site occurs between 10 and 12 hpi. (C) Binary images of the red channel of a PGE2-treated larva at 0 and 2 hpc. At 12 hpi, neutrophils have migrated away from the wound site, when treated with PGE2. (D) Plot showing the number of neutrophils moving away from the wound over 10 to 12 hpi, preincubated with or without PGE2 from 8 to 9 hpi. PGE2-treated neutrophils migrate away from the site of injury between 10 and 12 hpi more readily. Line of best fit shown is calculated by linear regression. P value shown is for the difference between the two slopes. (E) The speed of neutrophils moving away from the site of injury is not significantly different in the presence of exogenous PGE2, indicating that neutrophils migrate away sooner rather than at a greater speed in PGE2-treated larvae. (F) Neutrophil counts at 24 hpi show a significant increase in neutrophil number when EP4 signaling is blocked using the antagonist AH23848 **P < 0.01, ****P < 0.0001. Addition of PGE2 does not lead to significant abrogation of this effect, implying PGE2 signals through the EP4 receptor to promote neutrophil removal. All data are presented as means ± SEM, from n = 18 larvae for (D) and (E) and n = 53 for (F) from three experimental repeats. Images were taken using ×10 magnification on a TE2000U inverted microscope (Nikon). EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|