- Title
-
Nodal signaling has dual roles in fate specification and directed migration during germ layer segregation
- Authors
- Liu, Z., Woo, S., Weiner, O.D.
- Source
- Full text @ Development
|
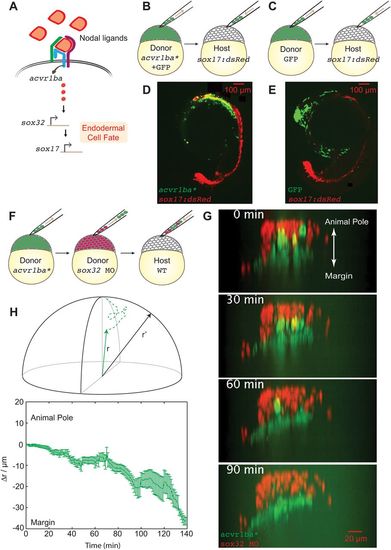
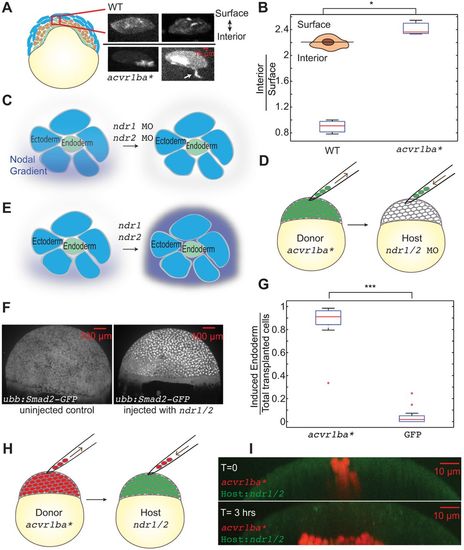
Constitutively active Nodal receptor (acvr1ba*)-induced ectopic endodermal cells sort into the inner layer of the embryo by ingression. (A) Schematic depicting Nodal signaling and specification of endodermal cell fate. Nodal ligands activate the Acvr1ba receptor and signal to sox32, a transcription factor controlling endodermal specification. (B-E) Schematics of the ectopic endoderm transplant assay (B,C) and representative results (D,E). acvr1ba*-expressing or control cells were transplanted to the animal pole of Tg(sox17:dsRed) host embryos. At 21-somite stage, transplanted acvr1ba*-expressing cells localized to endoderm-derived tissue, primarily the pharynx (D), whereas control transplanted cells localized to non-endodermal tissue, particularly the head (E). (F) Schematic of the double donor transplant assay. Donor endodermal cells expressing acvr1ba* (green) were transplanted together with non-endodermal donor cells injected with sox32 MO (red) to the animal pole of a single wild-type (WT) host. (G) Images from a time-lapse movie of a wild-type host containing both acvr1ba*-expressing (green) and sox32 MO-containing (red) donor cells. Time lapse microscopy began immediately after transplantation (0 min). Over time, sox32 MO donor cells remain in the outer layer of the embryo, whereas acvr1ba*-expressing donor cells migrate into the inner layer of the embryo. Data were re-sliced and projected onto the xz plane, with the animal pole towards the top and the margin towards the bottom. (H) Single-cell tracking analysis of ingression. Top: Cartesian coordinates for transplanted cells were transformed into spherical coordinates. Dashed lines represent cell trajectories. The radial distance, r, was measured as the distance from each cell's position at the end of the time-lapse movie to the center of the embryo (solid lines). r′ was measured as the distance to the host surface for normalization. Bottom: Average relative distance (±s.e.m.) of acvr1ba*-expressing cells plotted against time. Relative distance for each time point was calculated by measuring the radial distance of acvr1ba*-expressing cells to the center of the embryo, subtracted by the distance of host cell expanding during gastrulation. |
|
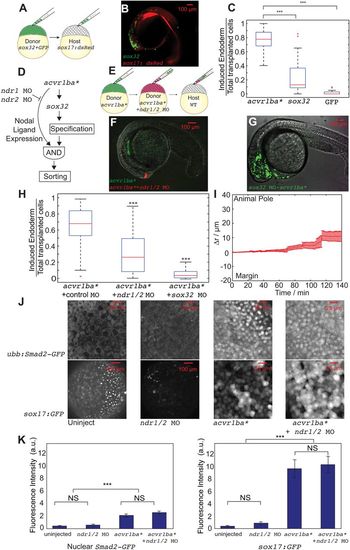
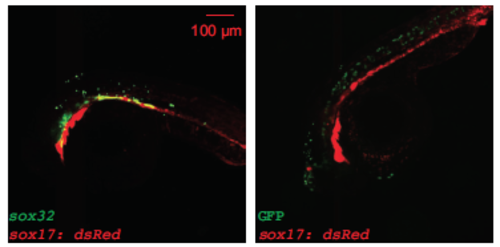
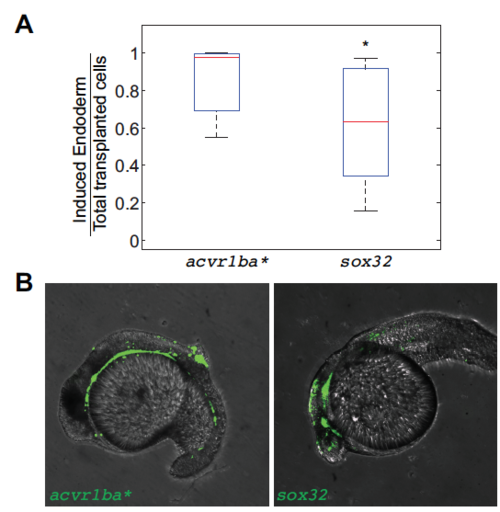
Nodal ligand expression is necessary to trigger the sorting of ectopic acvr1ba*-induced endodermal cells. (A,B) Schematics depicting sox32-induced ectopic endoderm transplant assay (A) and representative result (B). sox32-overexpressing cells were transplanted to the animal pole of wild-type host embryos. At the 21-somite stage, transplanted sox32-overexpressing cells primarily localized to non-endodermal tissues, primarily in the head and skin. (C) Boxplot quantification of endoderm contribution of transplanted cells at 20 hpf, assessed by colocalization with Tg(sox17:dsRed) expression. Here and in subsequent figures, for each box, the central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers, and the outliers are plotted individually using the ‘+’ symbol. Compared with acvr1ba*-expressing cells, fewer cells overexpressing sox32 contributed to endodermal tissues. Data are shown as mean±s.e.m. of three independent transplantation experiments with 26 embryos per condition. ***P<0.001 (Student's t-test). (D) Schematic depicting a potential AND gate for endoderm sorting. Constitutively activate acvr1ba* upregulates both sox32 as well as Nodal ligand expression. Only with both inputs do cells successfully sort to the inner layer of the embryo. (E) Schematic of the cell transplantation assay to test the necessity of Nodal ligand expression for cell sorting. Donor cells containing ndr1 and ndr2 MOs plus acvr1ba* mRNA (red) were transplanted together with cells overexpressing acvr1ba* only (green) into the animal pole of a wild-type (WT) host embryo. (F) Representative images showing the distribution of transplanted cells at the 21-somite stage. Cells expressing acvr1ba* only (green) localize to endoderm-derived tissue, primarily the pharynx. Cells containing acvr1ba* along with ndr1 MO and ndr2 MO (red) localize to non-endodermal tissue, primarily in the head. Lateral view, anterior to the bottom-left. (G) Representative image showing the distribution of transplanted cells at the 21-somite stage. Cells expressing acvr1ba* along with sox32 MO (green) localize to ectoderm-derived tissue, primarily the head. Lateral view, anterior to the bottom-left. (H) Boxplot quantification of endoderm contribution of transplanted cells at 20 hpf. ndr1 and ndr2 knockdown as well as sox32 knockdown reduced the ability of acvr1ba*-expressing cells to contribute to endodermal tissue. Data are shown as mean±s.e.m. of three independent transplantation experiments with 22 embryos per condition. ***P<0.001 (Student's t-test). (I) Single-cell tracking analysis of ingression of acvr1ba*-expressing cells with ndr1 MO and ndr2 MO. Average relative distance (±s.e.m.) plotted against time. Relative distance was calculated as in Fig. 1H. (J) Nodal signaling levels assessed by Tg(ubb:Smad2-GFP) and Tg(sox17:GFP). Top: Smad2-GFP showed no nuclear localization in cells at the animal poles (AP) of uninjected embryos and ndr1/2 morphants. Smad2-GFP showed comparable levels of nuclear localization in acvr1ba*-injected embryos and acvr1ba* with ndr1 and ndr2 MO-injected embryos. Bottom: Sox17:GFP labels wild-type endodermal cells in the uninjected control embryo but few GFP-positive cells are present in the ndr1/2 morphants. Sox17:GFP shows elevated level of expression in both acvr1ba*-injected embryos and acvr1ba* with ndr1 and ndr2 MO-injected embryos. Animal pole view. (K) Quantification of Nodal signaling level. Nuclear Smad2-GFP and Sox17:GFP fluorescence levels are quantified. Data are shown as mean±s.e.m. of three independent embryos. ***P<0.001 (Student's t-test). NS, not significant. |
|
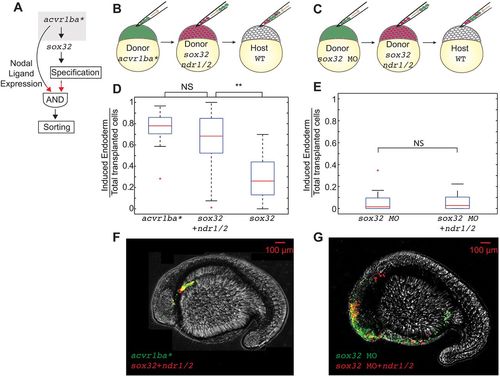
The combination of Nodal ligand expression and endodermal fate is sufficient to trigger ectopic endodermal cell sorting. (A) Schematic depicting putative AND gate for endoderm sorting. Red arrows demonstrate the experimental perturbation to test the sufficiency of Nodal ligands to induce ingression. (B,C) Schematics of double transplantation assay to test the sufficiency of the AND gate depicted in A for endodermal sorting. Cells overexpressing acvr1ba* (green) were transplanted together with cells overexpressing sox32, ndr1 and ndr2 (red) into the animal pole of a wild-type (WT) host embryo (B). Cells containing sox32 MO only were transplanted together with cells containing sox32 MO as well as ndr1 and ndr2 mRNAs were transplanted into the animal pole of a wild-type host embryo (C). (D) Boxplot quantification of endoderm contribution at 20 hpf of the transplanted cells depicted in B. Cells overexpressing sox32, ndr1 and ndr2 contributed to endoderm at a similar rate compared with cells overexpressing acvr1ba*. Data are shown as mean±s.e.m. of three independent transplantation experiments with 18 embryos per condition. **P<0.01 (Student's t-test). (E) Boxplot quantification of endoderm contribution at 20 hpf of the transplanted cells depicted in C. Neither cells containing sox32 MO nor cells containing sox32 MO and overexpressing ndr1 and ndr2 contributed to endodermal tissue. In addition, cells expressing acvr1ba* and sox32 MO did not contribute to endodermal tissue. Data are shown as mean±s.e.m. of two independent transplantation experiments with 14 embryos per condition. Student's t-test. (F) Representative image showing distribution of the transplanted cells depicted in D at the 18-somite stage. acvr1ba*-expressing cells localize to the endoderm-derived tissue, primarily the pharynx (green). Cells overexpressing sox32, ndr1 and ndr2 also localize to endoderm-derived tissue, primarily the pharynx (red). Lateral view, anterior to the left. (G) Representative image showing distribution of the transplanted cells depicted in E at the 18-somite stage. Cells expressing sox32 and Nodal ligands (ndr1, ndr1) localize to endodermal tissues similar to cells expressing acvr1ba*. In contrast, sox32 MO-injected cells (green) and cells injected with sox32 MO and ndr1 and ndr2 mRNAs (red) localize to non-endodermal tissue, primarily in the head and skin. Lateral view, anterior to the left. NS, not significant. |
|
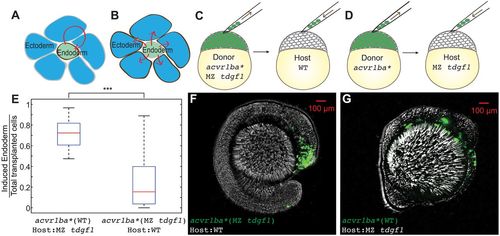
Nodal ligand reception acts cell-autonomously to support sorting. (A,B) Schematics depicting autonomous (A) versus non-autonomous (B) Nodal ligand reception (red arrows). (C) Schematic depicting single donor transplant assay to test cell-autonomous Nodal signal reception. acvr1ba*-expressing cells from MZ tdgf1 donor embryos were transplanted to the animal pole of a wild-type (WT) host embryo. (D) Schematic depicting single donor transplant assay to test non-cell-autonomous Nodal signal reception. acvr1ba*-expressing cells from wild-type donor embryos were transplanted to the animal pole of a MZ tdgf1 host embryo. (E) Boxplot quantification of endoderm contribution at the 18-somite stage for all transplanted cells. Wild-type donor cells expressing acvr1ba* contributed to endodermal tissues whereas acvr1ba*-expressing cells from MZ tdgf1 embryos did not. Data are shown as mean±s.e.m. of two independent transplantation experiments, with 14 embryos per condition. ***P<0.001 (Student's t-test). (F) Representative image showing distribution of MZ tdgf1 cells expressing acvr1ba* in a wild-type host. Donor cells (green) localized to ectoderm-derived tissue, primarily the head. Lateral view, anterior to the right. (G) Representative image showing distribution of wild-type cells expressing acvr1ba* in a MZ tdgf1 host. Donor cells (green) localized to endoderm-derived tissue. Lateral view, anterior to the right. |
|
Nodal ligands initiate but do not guide the ingression of endodermal cells. (A) Actin localization in ectopic endodermal cells. Blue, ectoderm; brown, mesoderm; green, endoderm. Donor embryos were injected with GFP-UTRN mRNA to label actin filaments. Cells overexpressing acvr1ba* or control cells expressing GFP-UTRN only were transplanted to the animal poles of wild-type host embryos. Actin was enriched on the interior side of acvr1ba*-expressing cells whereas control cells exhibited uniform actin distribution. Data were re-sliced and projected to the xz plane, with the surface of the embryo towards the top and the interior towards the bottom. Arrow shows interior-facing protrusion. (B) Boxplot of the ratio of interior to surface accumulation of actin. acvr1ba*-expressing cells exhibited significant interior enrichment of actin compared with control cells. Data are shown as mean±s.e.m. of three independent transplantation experiments, with 58 cells per condition. *P<0.05 (Student's t-test). Schematic shows the segmentation of surface versus interior of an endodermal cell. (C,E) Investigation into whether the endogenous Nodal gradient functions as a directional cue for endoderm ingression through knockdown of endogenous Nodal ligands. (C) ndr1 and ndr2 MOs were injected into host embryos to remove the endogenous Nodal gradient. (D) Cells expressing acvr1ba* were transplanted to the animal pole of a Nodal-depleted host. (G) Boxplot quantification of endoderm contribution at 20 hpf for all transplanted cells. Cells overexpressing acvr1ba* still contributed to endodermal tissues even in the absence of an endogenous Nodal gradient. Data are shown as mean±s.e.m. of two independent transplantation experiments, with 15 embryos per condition. ***P<0.001 (Student's t-test). (E) Saturating the endogenous Nodal gradient to test whether it acts as a directional cue. (F) Tg(ubb:Smad2-GFP) shows uniform nuclear translocation in a ndr1 and ndr2-injected embryo, suggesting uniform Nodal signaling. (H) Cells expressing acvr1ba* were transplanted to the animal pole of a Nodal-saturated host. (I) Representative image showing positions of acvr1ba* cells (red) immediately and 3 h after transplantation in a Nodal-saturated host. |
|
sox32-expressing cells preferentially segregate to endoderm-derived tissues when placed near the dorsal margin. Related to Figure 2. Representative images showing distribution of sox32-overexpressing cells or GFP-expressing cells that were transplanted to the margin of wild-type host embryos. At 21-somite stage, transplanted sox32-overexpressing cells primarily localized to endodermal tissues while GFPexpressing cells localized to mesodermal tissues. |
|
Induced endodermal cells internalize following transplantation to the margin. (B) Representative image showing distribution of transplanted cells depicted in (A) at 18 hpf. acvr1ba*-expressing cells localized to the endoderm-derived tissue (green). Cells overexpressing sox32 localize to both endoderm and ectoderm-derived tissue. Lateral view, anterior to the left.
|
|
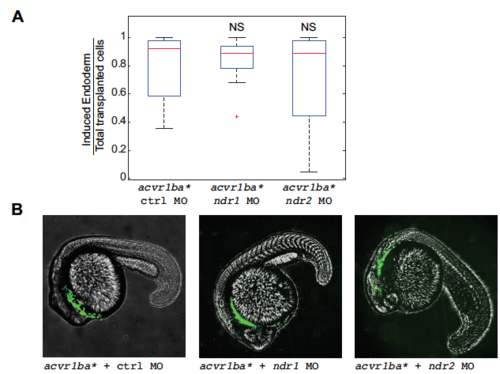
Ndr1 and Ndr2 act redundantly to support the ability of acvr1ba* cells to internalize. (A) Boxplot quantification of endoderm contribution of transplanted cells at 20 hpf. Data is shown as mean ± SEM of independent transplantation experiments with 16 embryos per condition. Student’s t-test was performed. NS, not significant. (B) Representative image showing distribution of transplanted cells depicted in (A) at 18 hpf. acvr1ba*-expressing cells localized to the endoderm-derived tissue (green)in all three conditions, in contrast to the block of internalization when both Ndr1 and Ndr2 MO are combined in acvr1ba* cells (Fig. 2H). Lateral view, anterior to the left. |
|
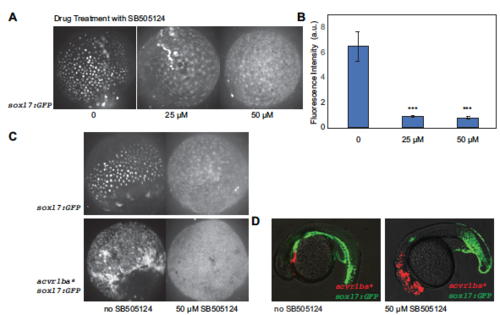
Nodal signaling inhibitor SB505124 blocks acvr1ba*-expressing cells from sorting. (A) Representative images of sox17:GFP expression under 0, 25 μM or 50 μM SB505124. Drug treatment began at 6 hpf, images were taken at 10 hpf. Animal pole view. (B) Quantification of sox17:GFP fluorescence intensity under 0, 25 μM or 50 μM SB505124. *** p<0.001. n=3. (C) sox17:GFP expression for embryos with or without injection of acvr1ba* and under no drug treatment or treated 50 μM drug SB505124 treatment. (D) Transplant of acvr1ba*-expressing cells into sox17:GFP background under DMSO control and 50 μM drug SB505124 treatment at 18hfp. |
|
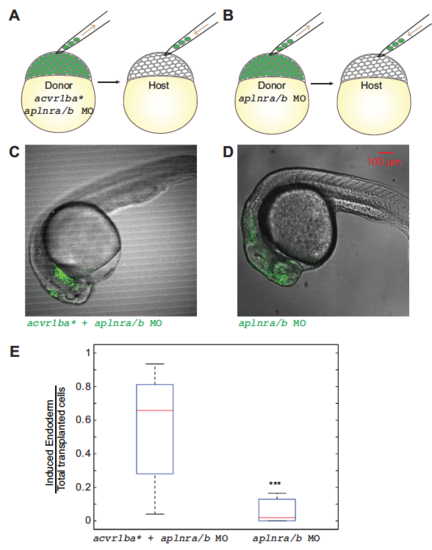
Apelin receptor signaling is not essential for ectopic endoderm ingression. (A-B) Schematic diagrams depicting single donor transplant assay to test the role of apelin receptor signaling. (A) acvr1ba*-expressing cells with aplnra and aplnrb MOs were transplanted to the animal pole of a wild-type host embryo. (B) Cells with aplnra and aplnrb MOs alone were transplanted to the animal pole of a wild-type host embryo. (C-D) Representative images showing distribution of induced endodermal cells in a wild-type host. Donor cells in (A) (green) mainly localized to endoderm-derived tissue (C), while donor cells in (B) mainly localized to ectoderm-derived tissue (D). Lateral view, anterior to the right. (E) Boxplot quantification of endoderm contribution at 21 hpf of transplanted cells depicted in (AB). acvr1ba*-expressing cells with aplnra and aplnrb MOs contributed to endoderm significantly more than cells with aplnra and aplnrb MOs alone. Data is shown as mean ± SEM of 3 independent transplantation experiments with 18 embryos per condition. Student’s t-test was performed. *** p<0.001. |