- Title
-
The VAMP-associated protein VAPB is required for cardiac and neuronal pacemaker channel function
- Authors
- Silbernagel, N., Walecki, M., Schäfer, M.K., Kessler, M., Zobeiri, M., Rinné, S., Kiper, A.K., Komadowski, M.A., Vowinkel, K.S., Wemhöner, K., Fortmüller, L., Schewe, M., Dolga, A.M., Scekic-Zahirovic, J., Matschke, L.A., Culmsee, C., Baukrowitz, T., Monassier, L., Ullrich, N.D., Dupuis, L., Just, S., Budde, T., Fabritz, L., Decher, N.
- Source
- Full text @ FASEB J.
|
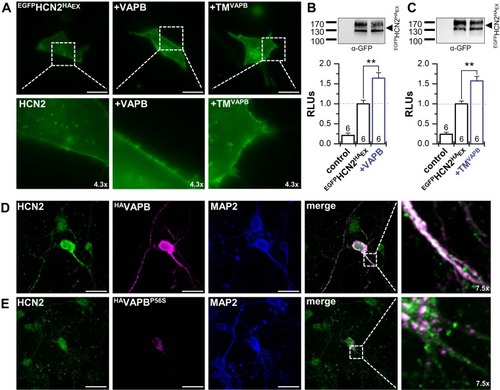
VAPB determines surface expression and dendritic localization of HCN2. |
|
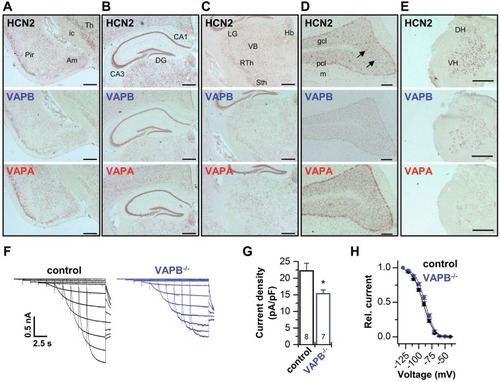
Codistribution of VAPs with HCN2 and contribution to thalamic |
|
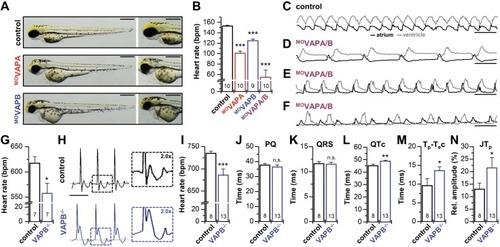
Bradycardia in knock-down zebrafish embryos and VAPB−/− mice. PHENOTYPE:
|
|
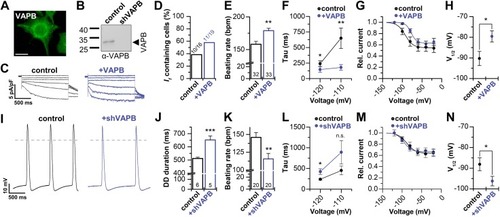
VAPB modulates |
|
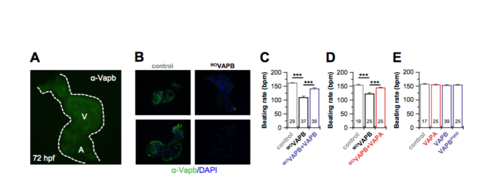
Cardiac expression of VAPB in embryonic zebrafish hearts, rescue experiments after MO-VAPB knock-down and overexpression of VAPBP56S. A) Immunostaining with an α-VAPB antibody (see Supplementary Methods) showing an uniform expression of VAPB in embryonic zebrafish hearts at 72 hours post fertilization. A, atrium. V, ventricle.B) Two representative embryonic zebrafish hearts stained against VAPB, either after injection with control morpholinos or with the VAPB knock-down morpholino, respectively. The embryonic zebrafish heart was co-stained against DAPI to show the cell nuclei. The experiments illustrate an efficient knock-down of VAPB and specificity of the antibody. C) After morpholino-antisense mediated VAPB knock-down (MOVAPB), the injection of cRNA encoding for either VAPB or D) VAPA rescued the reduction in embryonic heart rates. Data were obtained from four and three independent batches of injections, respectively. E) The injection of cRNA for the human VAPA or VAPB did not alter embryonic heart rates. Moreover, injection of VAPBP56S cRNA is likely to not act in a dominant-negative manner on zebrafish If channels, as the heart rates are not altered. Data were obtained from three independent batches of injections. All data are presented as mean ± s.e.m.. The number of experiments (n) are indicated in the bar graphs. ***, P < 0.001 using an unpaired Welch́s T-test. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|