FIGURE SUMMARY
- Title
-
Retinoic Acid Maintains Function of Neural Crest-Derived Ocular and Craniofacial Structures in Adult Zebrafish
- Authors
- Chawla, B., Swain, W., Williams, A.L., Bohnsack, B.L.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
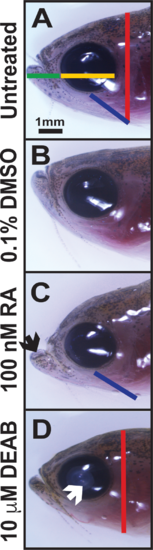
Alterations in RA affect craniofacial structures. Treatment of adult zebrafish with 100 nM RA (C) for 5 days caused a prognathic jaw (black arrow) and increased distance from the eye to the gill (blue line) as compared to untreated (A) and 0.1% DMSO control–treated (B) fish. Inhibition of RA synthesis through treatment with the pan–aldehyde dehydrogenase inhibitor DEAB (10 μM) for 5 days (D) decreased the height of the head (red line) and caused cataract formation (white arrow). Treatment with RA or DEAB did not affect the jaw length (green line, [A]) or horizontal (anterior to posterior) diameter of the cornea (yellow line, [A]).
|
|
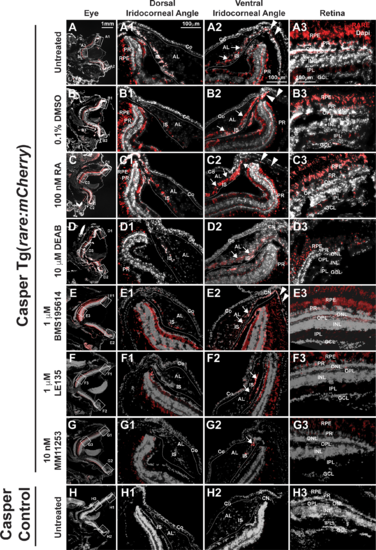
RA activity is in the retina and iridocorneal angles in the adult eye. In Casper Tg(rare:mCherry) adult zebrafish, RA activity was predominantly in the PRs and RPE in the adult eye of untreated (A, A3) and 0.1% DMSO control–treated (B, B3) fish. RA was also found in the OPL and IPL (A3, B3) in the retina. In the dorsal iridocorneal angle of untreated and control-treated eyes (A1, B1), RA activity was in the IS. In the ventral iridocorneal angle of untreated and control-treated eyes (A2, B2), RA was found in IS, the canalicular network that was between the iris and annular ligament (arrows), and the angular aqueous plexus (arrowheads). There was no detectable RA activity in the cornea (Co) in this reporter line. Treatment with 100 nM RA for 5 days (C) increased RA activity in the IS in the dorsal (C1) and ventral (C2) angles and in the ventral canalicular network (arrows, [C2]), but not in the retina (C3). Treatment with 10 μM DEAB for 5 days (D) decreased RA activity throughout the eye including in the dorsal (D1) and ventral (D2) angles and in the retina (D3). Treatment with the RARα-specific antagonist BMS195614 (1 μM) for 5 days did not alter RA activity within the eye (E, E1, E2, E3). Treatment with the RARβ antagonist LE135 (1 μM, F) for 5 days showed mildly decreased RA activity in the retina in the PRs, OPL, and IPL (F3), but no change in the dorsal (F1) or ventral (F2) angles. Inhibition of RARγ with the specific antagonist MM11253 (10 nM) decreased RA throughout the eye (G), including in the angles (G1, G2) and retina (G3). Control Casper fish, which lack the transgene, showed minimal to no fluorescence in the eye (H, H1, H2, H3). AL, annular ligament; GCL, ganglion cell layer; INL, inner nuclear layer; ONL outer nuclear layer.
|
|
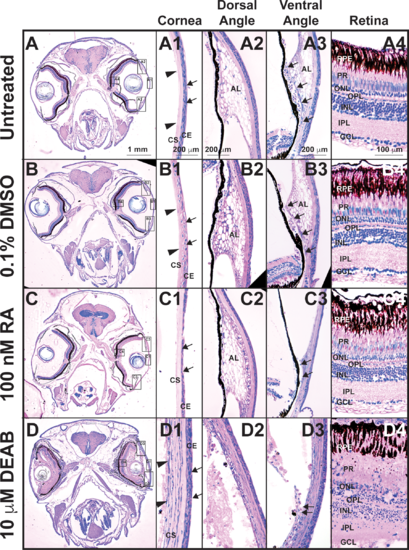
Tight control of RA levels is required for maintaining ocular structures. Methylacrylate sections of adult zebrafish treated with 100 nM RA for 5 days showed that exogenous RA decreased corneal epithelial cellularity (arrows, [C, C1]) as compared to untreated (A, A1) and DMSO control–treated (B, B1) fish. In the ventral iridocorneal angle (C3), RA also caused decreased iris stromal cellularity (C3) and loss of the webbed appearance of the AL and the ventral canalicular network (arrows, [C3]) compared to untreated (A3) and DMSO control-treated (B3) fish. The structures in the dorsal iridocorneal angle (C2) and retina (C4) were not affected by exogenous RA as compared to untreated (A2, A4) and DMSO control–treated (B2, B4) fish. Treatment with 10 μM DEAB for 5 days caused corneal stromal (arrowheads, [D, D1]) and epithelial (arrows, [D1]) edema and loss of architecture in the dorsal (D2) and ventral (D3) iridocorneal angles. DEAB also disrupted retinal structure, which included loss of PRs (D4) and ganglion cells (GCL, D4). AL, annular ligament; Co, cornea; GCL, ganglion cell layer; INL, inner nuclear layer; ONL outer nuclear layer.
|
|
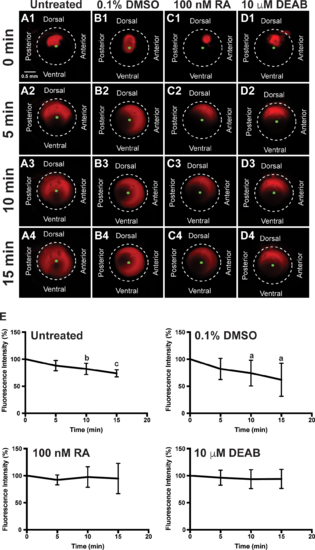
RA regulates aqueous outflow from the anterior segment of the eye. In vivo analysis of aqueous outflow showed that fluorescent Texas red dye injected into the anterior chamber diffused clockwise and counterclockwise toward the ventral iridocorneal angle over 15 minutes in untreated (A1–A4) fish. The fluorescence intensity of the dye did not change at 5 minutes (88.3% ± 9.6%), but was significantly decreased at 10 minutes (82.1% ± 10.4%) and 15 minutes (73.9% ± 6.7%) after injection (E). Similarly, the fluorescent dye diffused and exited the eye in fish treated for 2 days with 0.1% DMSO control (B1–B4). Fluorescence intensity was not changed at 5 minutes (81.8% ± 19.6%), but was significantly decreased at 10 minutes (74.3% ± 23.8%) and 15 minutes (62.0% ± 30.5%) after injection in control-injected fish (E). Fish treated with 100 nM RA for 2 days showed initial diffusion of the dye (C1), but no significant decrease in dye intensity at 5 minutes (92.3% ± 9.1%; [C2]), 10 minutes (97.6% ± 19.0%; [C3]), and 15 minutes (94.9% ± 28.1%; [C4, E]). Fish treated with 10 μM DEAB for 2 days also demonstrated initial dye diffusion (D1), but no significant decrease in dye intensity at 5 minutes (96.3% ± 13.8%; [D2]), 10 minutes (93.7% ± 17.6%; [D3]), and 15 minutes (94.1% ± 17.6%; [D4, E]).
|
|
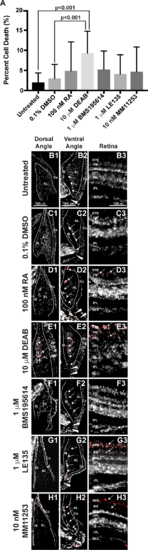
RA regulates cell survival in the anterior segment and retina. TUNEL assay showed that 2-day treatment with 10 μM DEAB (9.3% ± 5.4%) significantly increased the percentage of apoptotic cells (A) within the adult zebrafish eye as compared to untreated (2.0% ± 2.4%, P < 0.001) and 0.1% DMSO control–treated fish (3.0% ± 3.5%, P < 0.001). Two-day treatment with 100 nM RA (4.8% ± 7.3%), 1 μM BMS195614 (4.1% ± 4.8%), 1 μM LE135 (5.2% ± 4.6%), or 10 nM MM11253 (4.6% ± 6.2%) did not significantly change the percentage of apoptotic cells in the zebrafish eye. Despite no significant change in percentage of apoptotic cells in the eye, apoptosis was localized in RA-treated fish to the IS, canalicular network (arrows), and aqueous plexus (arrowheads) in the ventral iridocorneal angle (D2) as compared to untreated (B2) and DMSO control–treated (C2) fish. RA also showed increased apoptosis within the retinal pigment epithelium (arrows, [D3 compared to B3 and C3]), but did not affect cell survival in the dorsal iridocorneal angle (D1 compared to B1 and C1). Treatment with DEAB (10 μM) for 2 days caused diffuse apoptosis in the dorsal (E1) and ventral (E2) iridocorneal angles and in the retinal pigment epithelium and photoreceptors of the retina (arrows, [E3]). Treatment with the RARα-specific antagonist BMS195614 (1 μM) for 2 days did not affect cell survival in the dorsal angle (F1), ventral angle (F2), or the retina (F3). Inhibition of RARβ with LE135 (1 μM) for 2 days did not increase apoptosis in the angles (G1, G2) but did increase apoptosis in the retina (arrows, [G3]). The RARγ antagonist MM11253 (10 nM) caused localized apoptosis in the ventral angle (H2) and retina (H3), but not the dorsal angle (H1). AL, annular ligament; Co, cornea; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
|
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Invest. Ophthalmol. Vis. Sci.