- Title
-
Slow calcium waves mediate furrow microtubule reorganization and germ plasm compaction in the early zebrafish embryo
- Authors
- Eno, C., Gomez, T., Slusarski, D.C., Pelegri, F.
- Source
- Full text @ Development
|
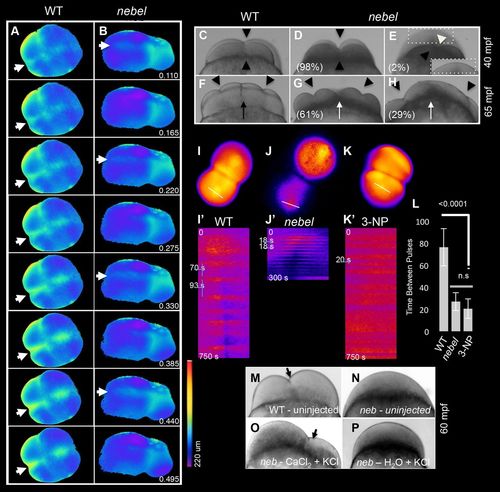
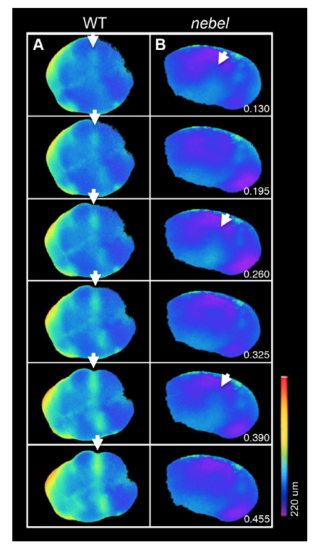
Reduced calcium release underlies furrow formation defects in zebrafish nebel mutants. (A,B) Fluorescence after injection of Fura-2 during second cleavage furrow formation, with calcium-saturated (340 nm) and calcium-free (380 nm) images converted to a pseudocolor ratio. Intracellular calcium increases are observed prior to and during furrow formation in wild-type embryos (A), whereas nebel mutants show repeated intracellular increases of reduced intensity (B). Animal views, with numbers indicating the lapsed fraction of the cell cycle. Arrows indicate sites of high intracellular calcium, corresponding to SCWs. (C-H) In nebel mutants, furrow initiation appears normal [black arrowheads during the first (D,E versus C) and third (G,H versus F) cell division]. In rare cases, the furrow fails to initiate (white arrowhead in E; insert depicts a clearer focal plane). Black arrow (F) indicates the adhesive septum in wild type, which fails to form in mutants (G,H, white arrows). (I-L) Ratiometric analysis using Oregon Green 488 BAPTA-Dextran and Rhodamine in wild-type (I), nebel mutant (J) and 3-NP-treated wild-type (K) embryos, with representative kymographs (I′-K′, white line in I-K) and average time in seconds between calcium pulses (L, n>6 furrows). Error bars indicate the s.e.m.; two-tailed P-values were assessed using an unpaired t-test. (M-P) Pseudocleavages in unfertilized wild-type eggs (M, arrow) do not form in nebel mutant eggs (N) but are induced by CaCl2 injection (O), an effect independent of the KCl co-injected to improve egg survival (P). |
|
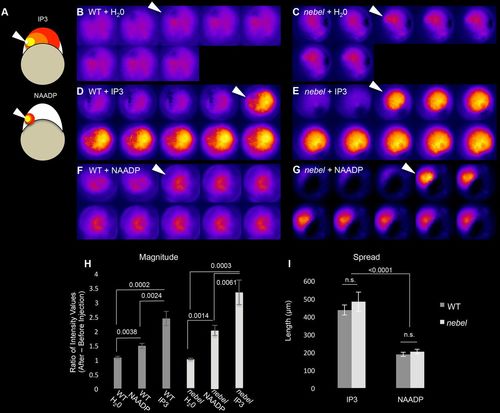
nebel mutants exhibit a normal response to calcium release agonists. (A) Experimental design. (B-G) IP3 or NAADP triggers a normal calcium release response in nebel mutants as compared with wild type. Agonist injection (arrowhead) was at 15-30 mpf, with time relative to injection indicated in min:s. (H,I) Quantification of magnitude and spatial extent of calcium release. Error bars indicate s.e.m. P-values according to paired t-test. |
|
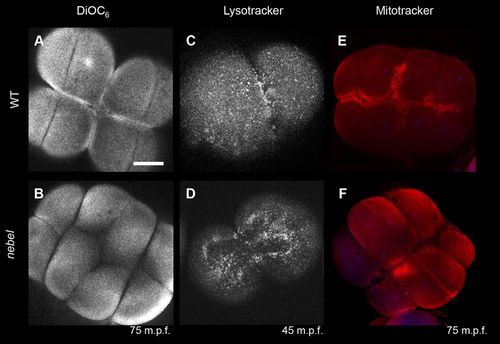
Calcium stores are not properly enriched at the furrows in nebel mutants. Furrow enrichment of (A,B) ER (5/5 wild type, 0/9 nebel mutant), (C,D) lysosomes (6/6 wild type, 2/7 nebel mutant) and (E,F) mitochondria (13/14 wild type, 2/18 nebel). Images are from live (A-D) and fixed (E,F) embryos. Scale bar: 100 μm in A for A-F. PHENOTYPE:
|
|
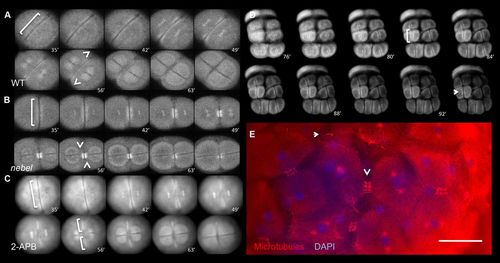
The FMA acts as a calcium-dependent modified midbody. (A-D) EMTB::EGFP transgenic fish. In wild type (A), the FMA initially aligns along the furrow (brackets) and becomes enriched distally (arrowheads). In nebel mutants (B), the FMA is initially normal but bundles in medial furrow regions. In 2-APB-treated wild-type embryos (C), the FMA forms normally but remains aligned along the furrow. During the early cleavage stages of wild-type EMTB::EGFP embryos (D), the FMA forms (bracket) but bundles medially (arrow). Time is in min. (E) Fixed wild-type 64-cell embryos stained for microtubules and for DNA (DAPI), showing inwardly bundling FMAs (arrowhead) and remnants (arrow). Scale bar: 75 μm in E. |
|
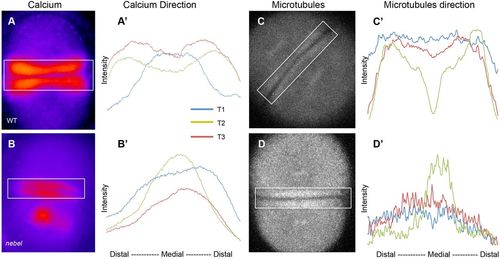
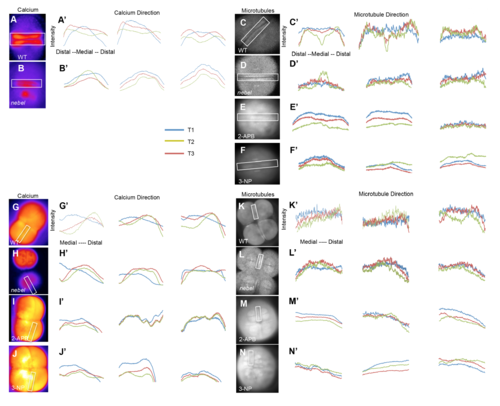
FMA distal enrichment correlates with SCW directionality. (A-B′) Calcium levels in single Tg[βactin2:GCaMP6s] transgenic embryos during furrow progression, with plotted profiles (A′,B′) corresponding to boxed areas. Wild-type embryos (A′), but not nebel mutants (B′), show increasing distal calcium levels. (C-D′) Microtubules in EMTB::EGFP transgenic embryos during furrow progression (C,D), with plotted profiles (C′,D′). Wild-type embryos (C′), but not nebel mutants (D′), show distal enrichment. Time points T1, T2 and T3 correspond, respectively, to frames within one-tenth of the total sequence of acquired images spanning a furrow formation cycle at the beginning, middle and end of that cycle. See Fig. S4 for additional profiles. |
|
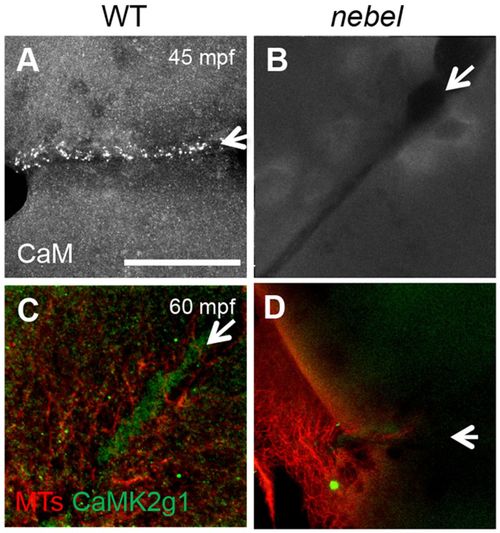
Calmodulin/CaMK is recruited to the furrow in wild-type but not in nebel embryos. Wild-type furrows show localization of Calmodulin (A, 5/7) and CaMK (CaMK2γ1; C, 13/15). nebel mutants lack this localization (B, 0/15; D, 0/10). Arrows indicate furrows. Scale bars: 10 μm in A for A-D. |
|
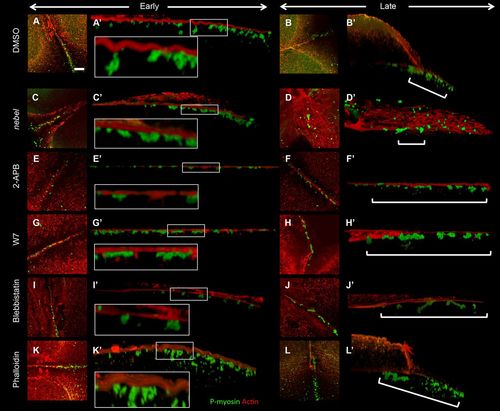
Furrow F-actin contractions associated with germ plasm RNPs depend on calcium, Calmodulin and myosin. (A-L) z-projections from early and late furrows (second and first furrows at 60 mpf, respectively). (A′-L′) Lateral views showing F-actin contractions and RNPs. Control treated embryos exhibit F-actin indentations (A,A′, 5/7) and subsequent RNP distal enrichment (B,B′, 4/5). nebel mutants and 2-APB-treated wild-type embryos exhibit reduced F-actin contractions (C,C′, 2/10) and failed distal RNP enrichment (D,D′, 3/10). (E-J) Various inhibitors result in reduced F-actin indentations and RNP distal enrichment: IP3 receptor inhibitor 2-APB (E-F′, 1/5, 0/4); Calmodulin inhibitor W7 (G-H′, 0/4, 0/4); myosin inhibitor blebbistatin (I-J′, 2/5, 2/7). Phalloidin-treated embryos exhibit F-actin indentations (K,K′, 5/8) but late furrows exhibit incomplete RNP distal enrichment (L,L′, 0/14). Insets (A′,C′,E′,G′,I′,K′) are magnifications of the boxed regions. Brackets (B′,D′,F′,H′,J′,L′) highlight regions of RNP distribution. Scale bar: 10 μm in A for A-L. |
|
Intracellular calcium release in wild-type and nebel mutant embryos. (A,B) SCWs at the third cleavage furrow (arrow) visualized by injection of the ratiometric calcium-sensitive Fura-2 dye, as in Fig. 1A,B, showing a reduced yet repeated pattern of intracellular calcium release in nebel mutants (B). Details as in Fig. 1A,B. |
|
Correlation of SCWs directionality and FMA movement. (A-B’,G-J’) Representative intensity profiles of SWCs (using injected OGB/Rhodamine (E-H’) and the FMA (using the EMTB::EGFP transgene (C-F’,K-N’)) in the first and second furrows for three embryos and at three different time points during furrow formation (see Fig. 7 legend) in untreated wild-type (A,C,G,K) and nebel mutant (B,D,H,L) embryos, and 2-APB- (E,I,M) and 3-NP-treated (F,J,N) wild-type embryos. Boxes in the overview image indicate region for intensity scan analysis. |
|
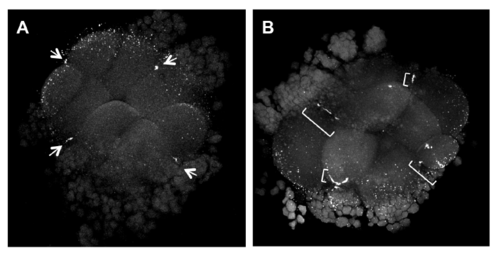
Rho inhibition results in defects in germ plasm RNP distal compaction. At a time (70 mpf) when germ plasm masses are highly compacted in distal furrow regions (A, arrows,12/12 furrows), embryos injected with the Rho inhibitor C3 exoenzyme show expanded RNP domains along the furrow (B, brackets, compacted in 4/20 furrows), indicative of defects in germ plasm distal compaction. |