- Title
-
Loss-of-function of the ciliopathy protein Cc2d2a disorganizes the vesicle fusion machinery at the periciliary membrane and indirectly affects Rab8-trafficking in zebrafish photoreceptors
- Authors
- Ojeda Naharros, I., Gesemann, M., Mateos, J.M., Barmettler, G., Forbes, A., Ziegler, U., Neuhauss, S.C.F., Bachmann-Gagescu, R.
- Source
- Full text @ PLoS Genet.
|
Vesicle accumulation is progressive from the onset of OS formation in cc2d2a-/- PRs (A-D) Transmission electron microscopy images (TEM) of retinal sections at 60 hpf are indistinguishable between wild-type (wt) (A, C) and cc2d2a-/- (B, D), including with respect to basal body docking (arrowheads in C-D) and extension of the connecting cilium (arrows in C-D). (E-H) Retinal sections at 72 hpf: note the nascent outer segments (OS) in wt (E, G), but the quasi-absence of OSs in cc2d2a-/- with onset of apical accumulation of vesicular structures (F, H, arrowheads). (I-L) Retinal sections at 96 hpf: well-formed OSs are present apical to the mitochondrial cluster in wt (I, K), while in mutant photoreceptors an increased number of vesicles (brackets) are found in the apical portion of the cell together with misshapen membrane stacks (arrowheads) instead of OSs (J, L). Scale bars are 1 μm in all panels. m mitochondria, N nuclei, OS outer segments, wt wild-type, hpf hours post fertilization. PHENOTYPE:
|
|
Accumulated vesicles in cc2d2a-/- PRs are opsin-carrier-vesicles (A-C) 5 dpf correlative light and electron microscopy (CLEM) image of retinal sections stained with BODIPY (magenta) to mark membranes of the outer segment and the mitochondrial cluster and with 4D2 (green) to label rhodopsin and red-green cone opsin. (A’-C’) are the corresponding scanning electron microscopy (SEM) images. Note that while 4D2 staining only localizes at the outer segments of wild-type (wt) PRs (A), it is visible in accumulated vesicles in cc2d2a-/- PRs (B, arrows) and in dysmorphic outer segments (C). Also note normal cilium docking in cc2d2a-/- PRs (C-C’, arrowhead). Scale bars are 2 μm in all panels. m mitochondria, N nuclei, OS outer segments, P pigment in melanosomes, wt wild-type. |
|
Rab8a and Rab8ba co-localize with endogenous opsins (A-C) 5 dpf cryosections of wild-type (wt) zebrafish expressing mCherry-tagged Rab8a in rods (magenta), stained with the anti-opsin antibody 4D2 (green). (D-F) 5 dpf cryosections of wt zebrafish expressing mCherry-tagged Rab8a in cones (magenta), stained with anti-blue opsin (green). (G-I) 5 dpf cryosections of wt zebrafish expressing mCherry-tagged Rab8ba in rods (magenta), stained with the anti-opsin antibody 4D2 (green). Arrowheads indicate examples of co-localization in all cases. Scale bars are 5 μm in all panels. EXPRESSION / LABELING:
|
|
Rab8 and opsin are associated with membrane-bound vesicular structures (A) CLEM image of wild-type (wt) transgenic zebrafish at 3 dpf expressing mCherry-tagged Rab8a in rods stained with anti-mCherry (magenta) and anti-opsin antibody 4D2 (green).(A’) High magnification image of the boxed area in (A) showing a clearly individualizable small round membrane-bound structure (white circle) coated with Rab8a signal on either side (empty arrowheads over the magenta signal) and with opsin signal over the edge of the structure (asterisk over the 4D2 signal), compatible with transmembrane opsins in a Rab8-coated vesicle. (A”) Immunohistochemistry image only from (A’). The white circle is placed where the vesicular structure is observed in the SEM image. (A”‘) SEM image only from (A’), showing the vesicular structure. The empty arrowheads are placed where the Rab8a signal is observed and the asterisk is located over the opsin signal. The additional m-Cherry and opsin signal likely represent a conglomerate of several vesicular structures with multilobulated membranes (arrows in A’ and A”‘). Scale bars: 4 μm in A and 1 μm in A’-A”‘. m mitochondria, N nuclei, OS outer segments, P pigment, wt wild-type. EXPRESSION / LABELING:
|
|
Rab8a partially mislocalizes in cc2d2a-/- cones 5 dpf CLEM images of wild-type (wt) (A-A’) and cc2d2a-/- (B-C’) zebrafish expressing mCherry-tagged Rab8a in cones (tg(tacp:mCherry-rab8a), magenta). The mCherry signal is enhanced with anti-mCherry antibody. (A-A’) Note the localization of Rab8a (magenta) to vesicular structures (arrowheads) in wt. (B-B’) In cc2d2a-/- cones, Rab8a (magenta) localizes to accumulated vesicles (v, bracket) as well as to non-membrane-delimited cytoplasmic areas (arrows in C-C’). Scale bars are 3 μm in all panels. OS outer segment, m mitochondria, N nuclei, v vesicular structures, wt wild-type. |
|
Rab8-particles display dynamic movement patterns and transiently approach the BB (A-B) Time-lapse imaging of a 5 dpf wt zebrafish retina expressing Rab8ba in rods (tg(rhod:mCherry-rab8ba)). The Rab8 particle marked with an arrow in all time frames can be recognized and followed by the Ilastik tracking software (cyan overlay) (A’-B’). This particle transiently approaches the GFP-tagged basal body (C and merge in D). The movement is specific for Rab8 as transiently expressed Rab3aa in rods (rhod:mCherry-rab3aa) (E-F’) exhibits the expected synaptic localization (F’). Scale bars are 10 μm in all images. OS outer segment, IS inner segment, Syn Synapse. EXPRESSION / LABELING:
|
|
SNAP25 mislocalizes in cc2d2a-/- PRs (A-C’) 4 dpf cryosections of wild-type (wt) (A-A’), cc2d2a-/- (B-B’) and ift88-/- (C-C’) retinae stained for SNAP25 (green in A’-C’) and BODIPY (magenta). In wt PRs (A-A’) SNAP25 localizes along the plasma membrane, between the mitochondrial cluster and the OS (arrowhead) and at the synapse. In cc2d2a-/- (B-B’), SNAP25 synaptic localization is preserved but apical mislocalization to a membrane-rich compartment (BODIPY, arrows) is obvious. (C and C’) Despite absence of OSs in the ift88-/- mutant, SNAP25 localizes correctly at the apical membrane of PRs. Scale bars are 10 μm in all panels. |
|
SNAP25 localizes to wt periciliary membrane and mislocalizes to accumulated vesicles of cc2d2a-/- PRs (A-E) 5 dpf CLEM retinal sections stained for SNAP25 (green), acetylated tubulin (magenta) and DAPI (blue, nuclei). (C-E) are higher magnification images of the boxed areas in (A, B) and (C’-E’) are the corresponding SEM images. In wt PRs (A) SNAP25 is found at the inner segment apical membrane, along the calycal processes (empty arrowhead in A) as well as at the periciliary membrane (C-C’, arrow) around the base of the anti-acetylated tubulin-marked cilium (C-C’, arrowhead). In contrast, in cc2d2a-/- PRs (B), SNAP25 prominently mislocalizes in accumulated vesicles (D-D’, arrows) and dysmorphic OSs (E-E’, bracket). Arrowheads point to connecting cilia in (C-D’). Scale bars: 4 μm in A-B, 1 μm in C-E’. OS outer segment, m mitochondria, N nuclei, P pigment, v vesicles, wt wild-type. |
|
Proteins involved in OCV fusion are affected by loss of Cc2d2a function 4 dpf wild-type (wt) (A-A’) and cc2d2a-/- retinal cryosections (B-B’) and 6 dpf wt (C-C’) and cc2d2a-/- retinal cryosections (D-D’), all stained for Syntaxin3 (Stx3) (grayscale in A-D and green in A’-D’) and counterstained with DiO (A’,B’) or BODIPY (C’,D’) (both magenta) to label membranes. In wt PRs at both developmental times Stx3 localizes along the plasma membrane (arrowhead), between the mitochondrial cluster and the OS and at the synapse (A-A’, C-C’), similar to SNAP25. While minimal Stx3 mislocalization is visible in cc2d2a-/- at 4dpf, a striking decrease in fluorescence intensity is obvious in the mutant (B, D). (E) Western blot on whole eye lysates at 6 dpf confirms decreased protein levels of Stx3 in cc2d2a-/-. Protein levels of SNAP25 and the Exocyst component Exoc4 are also decreased in cc2d2a-/- whole eyes. (F) Relative protein content was determined as the ratio of band intensity relative to the housekeeping protein control (beta-actin), averaged for all replicates and repeated in 3 independent blots. Error bars represent standard deviation. *p<0.05, ** p< 0.01, Student’s t-test. Full western blots are shown in S12 Fig. |
|
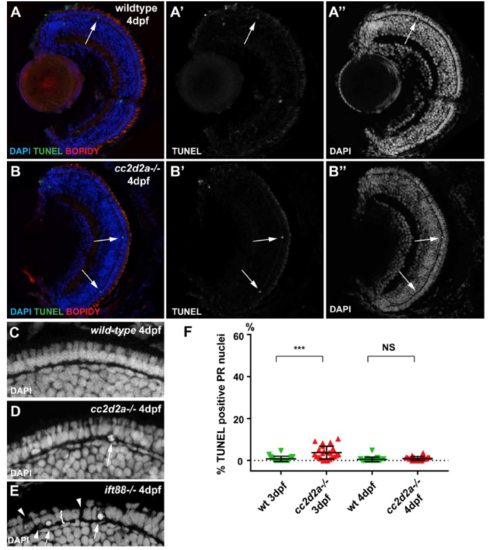
cc2d2a-/- retinae do not undergo degeneration at early developmental stages. (A-B”) TUNEL assay on 4 dpf retinal cryosections of wild-type (A-A”) and cc2d2a mutants (B-B”). Note the limited number of TUNEL positive cells (B’ and green in B) in mutant retinae. Membranes are counterstained with BODIPY (red in A-B) and nuclei with DAPI (A”-B” and blue in A-B). Also note the normal organisation of mutant retina, including the photoreceptor (PR) cell layer, visible with DAPI in (B”) compared to wild-type in (A”). (C-E) Higher magnification views of the PR cell layer in wild-type (C), cc2d2a mutant (D) and ift88 mutant (E) 4 dpf larvae. Note the normal nuclear morphology in cc2d2a-/- PRs compared to wild-type and compared to the degenerating ift88 retina which displays rounded nuclei (arrows in E), gaps (arrowheads in E) and a globally thinned PR cell layer (bracket in E). (F) Quantification of TUNEL positive cells in wild-type (green inverted triangles) and in cc2d2a mutant (red triangles) at 3 and 4 dpf. While the amount of cell death is statistically significantly increased at 3 dpf in mutant compared to wild-type, it remains minimal (on average 4.6% of evaluated nuclei are TUNEL positive in mutants, compared to 0.8% in wild-type). At 4 dpf, no increase in cell death is observed in cc2d2a mutant retinae compared to wild-type. NS non significant, *** p<0.001, t-test, n>20 animals for each condition. Quantification was performed on confocal stacks of identical dimensions in wild-type and mutant. |
|
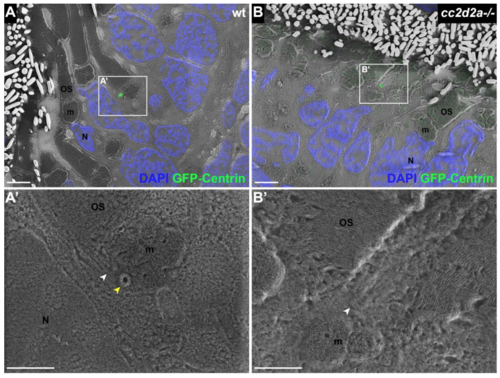
BB docking occurs normally in cc2d2a-/- PRs. 5 dpf CLEM sections of transgenic tg(tacp:GFP-hCentrin) wild-type (wt) (A) and cc2d2a-/- (B) fish expressing GFP-tagged centrin and counterstained with DAPI (blue, nuclei). GFP-Centrin-labeled basal bodies (BBs) (green) localize at the apical membrane of both wt and mutant animals. (A’) BB is docked right below the outer segment (white arrowhead), apical to the daughter centriole (yellow arrowhead) in wt. (B’) BB (white arrowhead) is localized correctly in cc2d2a-/- PRs even when the OSs appear dysmorphic and disorganized. Scale bars: 4 μm in A-B and 2 μm in A’-B’. OS outer segment, m mitochondria, N nucleus, wt wild-type. |
|
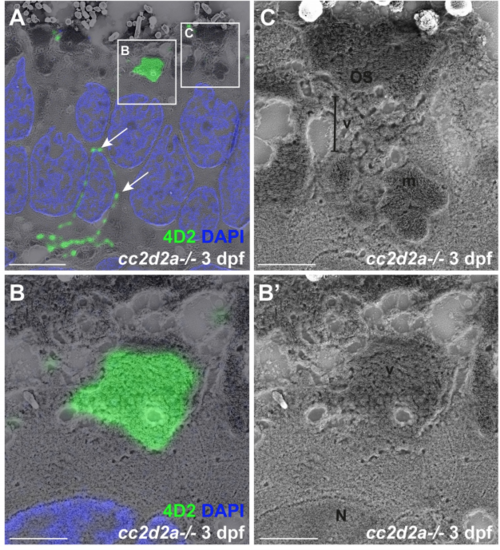
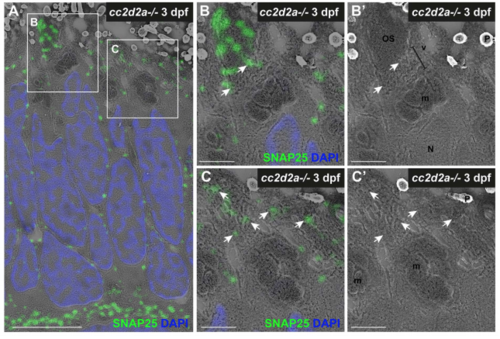
Accumulated vesicles in cc2d2a-/- PRs at 3 dpf contain opsin. (A) 3 dpf correlative light and electron microscopy (CLEM) image of a cc2d2a-/- retina stained with 4D2 (green) to label rhodopsin and red-green cone opsin and with DAPI (blue, nuclei). Arrows point to mislocalized opsin inside the cell body. (B-C) Higher magnification images of the boxed regions in (A). (B) Accumulated apical vesicles contain opsin in 4D2-positive PRs. (B’) corresponding scanning electron microscopy image only of (B). (C) Some stacking of membranes in OSs can be occasionally observed above accumulating vesicles. Scale bars: 4 μm in A and 1 μm in B-C. OS outer segment, m mitochondria, N nucleus, v vesicular structures. |
|
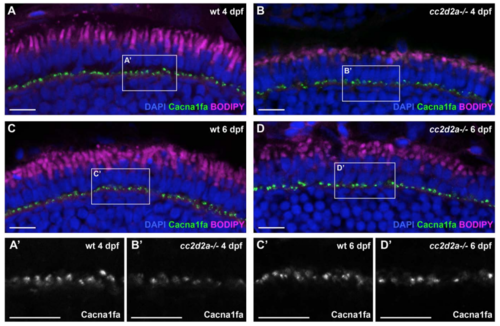
Polarized trafficking of transmembrane proteins not targeted to cilium occurs normally in cc2d2a-/- PRs. Retinal cryosections of 4 dpf (A-B) and 6 dpf (C-D) wild-type (wt) (A, C) and cc2d2a-/- (B, D) zebrafish, stained with an antibody against Cacna1fa (green), an L-type calcium channel that localizes to the synapse, and counterstained with DAPI (nuclei, blue) and BODIPY (outer segments and mitochondrial cluster, magenta). (A’-D’) Close-ups of the synaptic regions boxed in A-D. Cacna1fa (grey) in the mutant (B’ and D’) presents a similar localization at synapses as in wt (A’ and C’) at 4 and 6 dpf. Scale bars: 10 μm in all figures. |
|
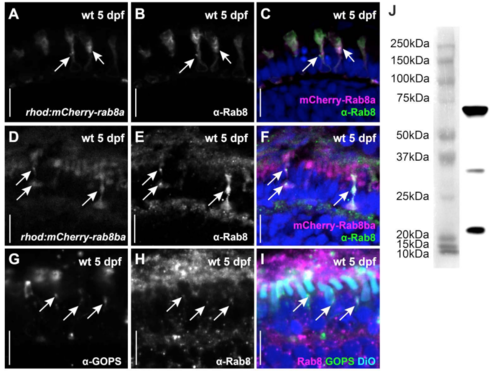
Comparison of transgenic Rab8 expression with endogenous Rab8 localization. An anti-Rab8 antibody (B, E and green in C, F) recognizes mCherry-tagged Rab8a (A, magenta in C) and mCherry-tagged Rab8ba (D, magenta in F) on retinal cryosections of 5 dpf zebrafish (arrows). The same antibody (H and magenta in I) recognizes endogenous Rab8 on non-transgenic tissue in a punctated pattern that localizes to the inner segment where it co-localizes with endogenous green opsin (G and green in I, arrows). Anti-Rab8 antibody signal is also very prominent over the retinal pigment epithelium (RPE) but not inside the DiO labelled OSs (cyan in I). Additional puncta are also visible at the synapse and between the nuclei. Such basal punctate localization pattern is partly consistent with mCherry localisation in the transgenic lines which is occasionally seen in such basal regions of the PRs (see time-lapse videos as well). Given that the transgenic lines analyzed express mCherry-Rab8 only in PRs, the relevance of the anti-Rab8 RPE signal cannot be evaluated. (J) A western blot of wild-type whole eyes at 5 dpf probed with the same anti-Rab8 antibody revealed a strong band with the expected size for Rab8 (23.57 KDa). In addition, two other bands were visible on western blot (also acknowledged by the manufacturer: https://www.novusbio.com/products/rab8a-antibody-3g1_h00004218-m02), indicating that the antibody may recognize additional epitopes. Thus, in the absence of Rab8a/Rab8ba/Rab8bb triple knockout mutants, it is not possible to determine the specificity of the anti-Rab8 RPE staining and of the additional puncta with certainty. Scale bars: 10 μm in all panels. |
|
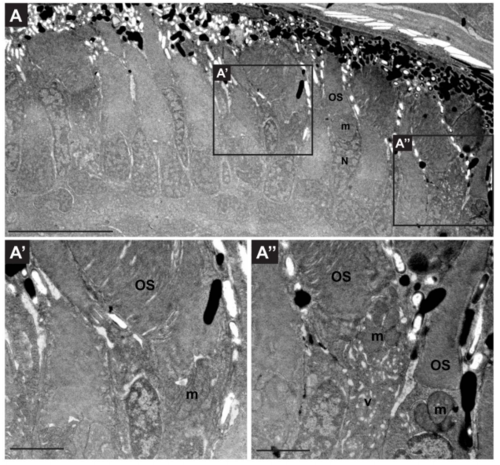
Retinae of rhod:mCherry-rab8a are structurally healthy. (A) Transmission electron microscopy image of a wild-type 5dpf tg(rhod:mCherry-rab8a) retina. The retinal structure remains normal and extension of outer segments is unaffected. Variable accumulation of membrane-bound structures in inner segment regions is observed in a subset of PRs as a consequence of the overexpression of the transgenic construct (absent in A’, present in A”). Scale bars: 10 μm in A and 2 μm in A’ and A”. OS outer segment, m mitochondria, N nucleus, v vesiculo-tubular structures. |
|
SNAP25 mislocalizes in OSs and accumulated vesicles of cc2d2a-/- PRs at 3 dpf. (A) 3 dpf correlative light and electron microscopy (CLEM) image of a cc2d2a-/- retina stained with anti-SNAP25 (green) and DAPI (blue, nuclei). (B-C’) Higher magnification images of the boxed regions in (A). SNAP25 mislocalizes in misshapen outer segments (B) and accumulated vesicles (C). (B’ and C’) are SEM images only of (B and C). Arrows point to vesicular structures where SNAP25 mislocalizes. Scale bars: 4 μm in A and 1 μm in B-C’. OS outer segment, m mitochondria, N nucleus, P pigment, v vesicular structures. |
|
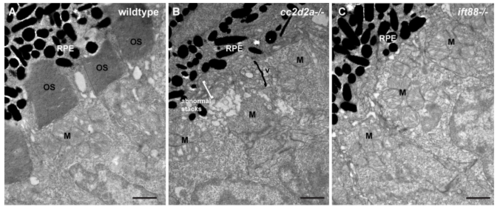
Comparison of cc2d2a and ift88 mutant retinae indicates that vesicle accumulation in PRs is not a general non-specific defect secondary to any ciliary dysfunction. (A-C) Transmission electron microscopy images of 3 dpf wild-type (A), cc2d2a mutant (B) and ift88 mutant (C) retinae. Note the accumulation of vesicular structures and abnormal membrane stacks in cc2d2a mutants, while no vesicles are found in the inner segments of ift88 mutants. |