- Title
-
The Retinol Binding Protein Receptor 2 (Rbpr2) is required for Photoreceptor Outer Segment Morphogenesis and Visual Function in Zebrafish
- Authors
- Shi, Y., Obert, E., Rahman, B., Rohrer, B., Lobo, G.P.
- Source
- Full text @ Sci. Rep.
|
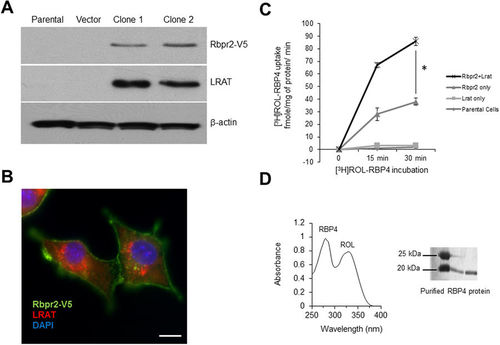
Zebrafish Rbpr2 mediates retinol uptake in NIH3T3 cells. (A) Western blot analysis confirmed co-expression of zebrafish Rbpr2 and human LRAT proteins in stable NIH3T3 clones. (B) Subcellular localization of zebrafish Rbpr2 (V5-tagged, green) and human LRAT (red) in stable NIH3T3 cells determined by immunohistochemistry and confocal microscopy. Nucleus, stained with DAPI (blue). Scale bar = 50 μm. (C) Parental NIH3T3 cells (diamond) or NIH3T3 cells expressing LRAT only (squares) or NIH3T3 cells expressing Rbpr2 only (triangle) or NIH3T3 cells co-expressing Rbpr2+LRAT (X) were incubated with [3H]ROL-RBP4. Cells were washed thrice and lyzed at the 0, 15 and 30 min time points. Protein concentrations were estimated and cells were subjected to scintillation counting. The x-axis shows the concentration of retinol-bound RBP4 taken up by the cells and expressed as fmole/mg of protein/min. (D) Representative images of absorption spectrum of recombinant RBP4-ROL at A280/330 nm. Inset, purified recombinant RBP4 protein, resolved by SDS-PAGE and visualized by Commassie Blue staining. *p < 0.005; [3H]ROL-RBP4 uptake values in NIH3T3 cells co-expressing Rbpr2 + LRAT compared to NIH3T3 cells expressing Rbpr2 receptor only. |
|
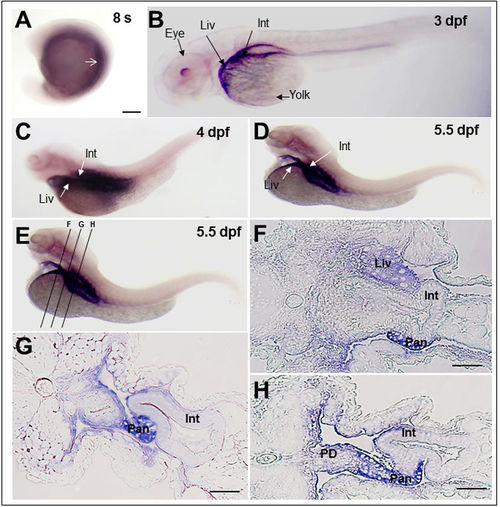
Rbpr2 mRNA expression patterns during zebrafish development analyzed by whole mount In-situ Hybridization (WISH). (A) At the 8-somite stage, zygotic rbpr2 mRNA (purple stain) is expressed in the yolk syncytium and in the mesendodermal cells. Staining is also detectable in the anterior somites (arrow). (B) At 3 days post fertilization (dpf), staining for rbpr2 mRNA expression (purple stain) becomes restricted and is observed in the developing liver (Liv) and Gut (Gut). At the 4 (C) and 5.5 dpf (D) larval stages, rbpr2 mRNA expression is maintained within the liver and intestine. (B–D) Interestingly, unlike zebrafish stra6 mRNA expression in the eye25, rbpr2 mRNA expression was not observed in the developing eyes. (E) Transverse sections through 5.5 dpf zebrafish larvae at three different regions, corresponding to panels F–H. (F–H) Histological analysis of transverse sections reveals rbpr2 mRNA expression (blue) in the liver hepatocytes (Liv) and intestinal enterocytes (Int). Pan, pancreas; PD, pancreatic duct; Liv, liver; Int, intestine. Scale bar = 100 μm (F–H). EXPRESSION / LABELING:
|
|
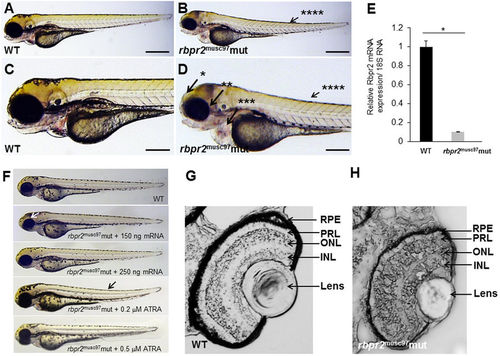
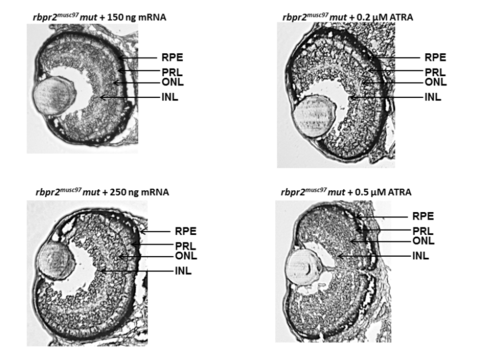
Rbpr2 musc97 mutants show eye and systemic phenotypes consistent with retinoid deficiency. Lateral view of representative WT (panels A,C) and rbpr2 homozygous mutant (rbpr2 musc97 mut, panels C,D) zebrafish at 5.5 dpf. Rbpr2 musc97 mutants showed gross defects, which included: *hydrocephaly; **smaller eyes, ***pericardial edema and ****slight tail curvature. Scale bars = 0.326 mm (A,B) and 0.103 mm (C,D). (E) qPCR analysis of rbpr2 mRNA expression from WT and rbpr2 musc97 mutant zebrafish larvae at 5.5 dpf. (F) Injection of WT zebrafish rbpr2 mRNA or dose specific treatment with all-trans retinoic acid (ATRA) rescues the rbpr2 musc97 mutant phenotype. Images obtained at 5 dpf. Rescue experiments of rbpr2 musc97 mutants with either mRNA or ATRA were repeated twice as outlined in methods. (G,H) Transverse sections of 5.5 dpf WT (panel G) and rbpr2 musc97 mutant (panel H) eyes. rbpr2 musc97 homozygous mutant eyes were smaller and show disruption of retinal lamination layers. PHENOTYPE:
|
|
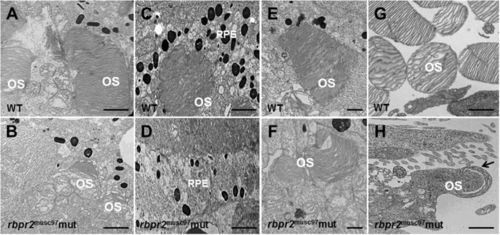
Ultrastructural analysis of WT and rbpr2 musc97 mutant photoreceptors. Transmission electron microscopy provided ultrastructural views of WT and rbpr2 musc97 mutant photoreceptor cells at 5.5 dpf. (A,C,E and G) WT photoreceptors exhibit tightly stacked outer segment membranes (panels A,C and E; arrows) and RPE cells containing many melanosomes (C); (B,D,F and G) while in rbpr2 musc97 mutants only remnants of outer segments (panels B and F; arrows) could be observed, with fewer melanosomes in the RPE cells (D). Scale bars = 800 nm (A–E); 400 nm (F); 200 nm (G and H). OS, outer segments; RPE, Retinal Pigmented Epithelium. |
|
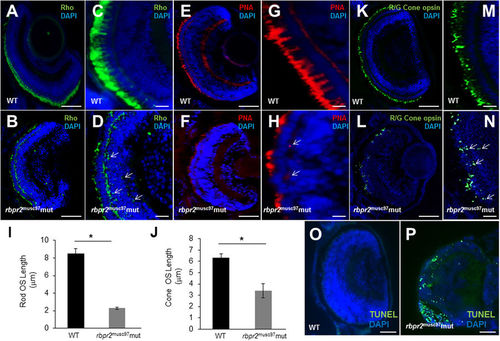
Immunohistochemical analysis of rod and cone photoreceptors in WT and rbpr2 musc97 mutant zebrafish. Rod photoreceptor outer segments were identified with 1D4 antibody specific for rhodopsin (green, Rho, panels A–D). Cone photoreceptors outer segments were identified with PNA-488 (red, PNA, panels E–H) and Red/Green Opsin antibody (green, R/G Cone opsin, panels K–N) all at 5.5 dpf. Opsin mislocalization was observed in rbpr2 musc97 mutants (indicated by white arrows in D,H and N). Severe loss of rod and cone pigment proteins was evident in the rbpr2 musc97 mutant zebrafish (B,D,F,H,L and N). Quantification of photoreceptor outer segment length at 5.5 dpf is provided for rods (I) and cones (J). TUNEL staining for apoptosis in WT (O) and rbpr2 musc97 mutant (P) zebrafish retinas at 6 dpf. TUNEL positive cells/apoptotic nuclei stain green. Scale bars: 100 μm (A,B,E,F,K,L,O and P); 25 μm (C,D,G,H,M and N). |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Eye sections of zebrafish at 5 dpf from rescue experiments Histological analysis of rbpr2musc97 mutant larvae eyes after rescue experiments at 5 dpf showed normal retinal lamination and eye patterning which were comparable to WT animals. PHENOTYPE:
|
|
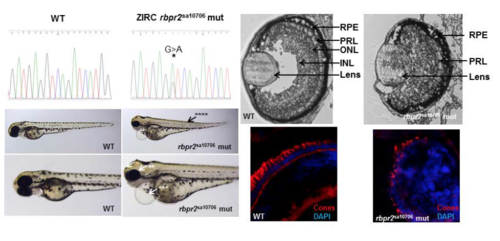
ZIRC rbpr2 mutants (rbpr2sa10706) show eye phenotypes consistent with retinol deficiency A rbpr2 mutant zebrafish line (G>A mutation; rbpr2sa10706) from the Zebrafish International Resource Center (ZIRC) which affects the essential splice site of exon 5/intron 6, was obtained and analyzed by light microscopy, histology and immunohistochemistry for cones at 5.5 dpf. The resulting eye phenotype observed was similar to the TALEN generated rbpr2 mutant phenotype (rbpr2musc97) described. Rbpr2sa10706 mutants showed gross defects, which included: *hydrocephaly; **smaller eyes, ***pericardial edema and ****slight tail curvature. |