- Title
-
Optical Control of Tumor Induction in the Zebrafish
- Authors
- Feng, Z., Nam, S., Hamouri, F., Aujard, I., Ducos, B., Vriz, S., Volovitch, M., Jullien, L., Lin, S., Weiss, S., Bensimon, D.
- Source
- Full text @ Sci. Rep.
|
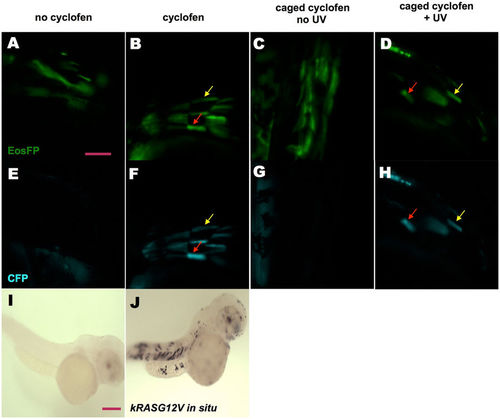
KRASG12V induction by incubation in cyclofen or photo-activation of caged cyclofen. (A,E) Injecting UAS:kRASG12V-T2A-CFP; ubi:Eos plasmid into Tg(ubi:Gal4-ERT2) embryos resulted in a mosaic expression of EosFP protein, but (in absence of free cyclofen) no expression of CFP. (B,F) Embryos treated with 2 μM cyclofen at 32hpf display expression of CFP co-localized with EosFP expression (denoted by arrows). (C,G) Incubation of caged cyclofen (with no UV) did not activate CFP expression while (D,H) 2 min UV uncaging effectively activated the expression of CFP 12 hours later (denoted by arrows). The induction of kRASG12V by cyclofen was confirmed by kRASG12V mRNA in situ hybridization. Human kRASG12V mRNA was transcribed only in the embryo incubated in cyclofen (J), but not in the control embryo (I). Scale bar: 200 μm. |
|
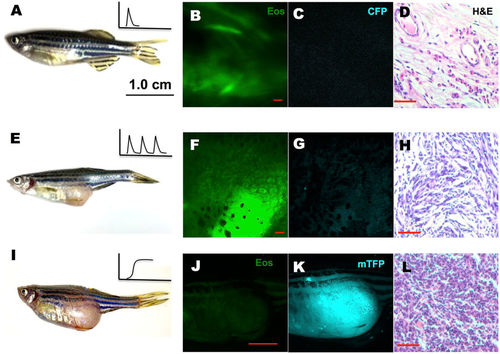
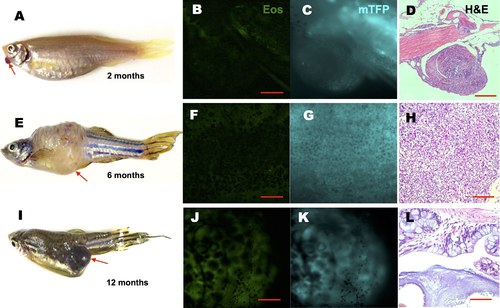
Tumor induced by periodic or constitutive, but not transient activation of kRASG12V expression. In the latter system, one-time activation of kRASG12V expression was induced by transient cyclofen incubation or photo-activation of caged cyclofen at 1dpf (A). Periodic kRASG12V expression was achieved by 1 day incubation in 2 μM cyclofen every 5 days for a course of two months (E). (B–D) Transient induction of kRASG12V did not affect normal development (as shown by the histopathological staining) with no CFP expression in one-year old fish. (E–H) Tumors were observed (albeit rarely) in fish periodically treated with cyclofen. A representative one-year old fish displayed a clear tumor (E) revealed by histopathological staining (H). Interestingly, the cells in the tumor still expressed EosFP (F), but lost CFP expression (G). (I–L) Constitutive kRASG12V expression following light-activation of caged cyclofen induced tumor (I) in 5 month-old fish as revealed by both fluorescent markers (J,K) and H&E staining (L). Scale bar: B, D, F, H, L 200 μm; J, 1 cm. |
|
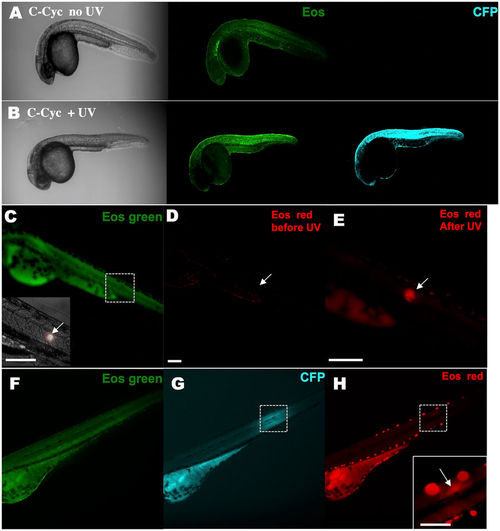
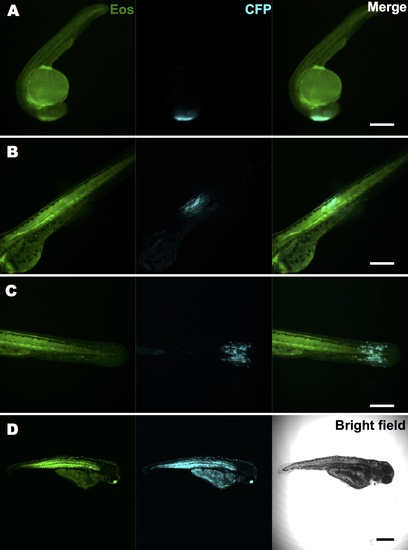
KRASG12V activation and tracking in a small group of cells within a live zebrafish embryo. A stable Tg(ubi:Eos;UAS:kRASG12V-T2A-CFP) line was generated and crossed with the Tg(ubi:Gal4-ERT2) line. Caged cyclofen alone at 1 dpf was not able to induce CFP expression (A), while 2-minute UV illumination (B) effectively turned on CFP after 18 hours. (C) Local activation of kRASG12V expression was achieved by introducing a focalized UV beam onto embryos pre-incubated with caged cyclofen. (D,E) 1 minute UV effectively converted EosFP from green to red. (F,G) After 18 hours, CFP expressed in a localized region of the illuminated embryo and weak remaining EosFP red signal was detected in the same region (H). Scale bar: 200 μm. |
|
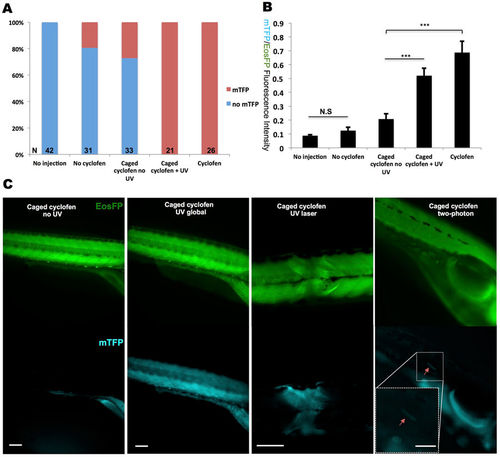
Constitutive photo-activation of kRASG12V expression in localized tissue and single cells. To induce constitutive activation of kRASG12V, Cre-ERT2 mRNA was injected into Tg(ubi:loxP-Eos-stop-loxP-kRASG12V-T2A-mTFP) embryos at the one-cell stage. (A) Despite some minor light independent activation of Cre, mTFP expression could be fully induced by both cyclofen and caged cyclofen photo-activation. (B) Quantification of kRASG12V activation signal via the ratio of the fluorescence of the co-expressed mTFP protein and the (partially) floxed Eos protein. (C) UV (lamp or laser) and two-photon activation were performed at 10hpf to achieve global (with the UV lamp), local (with the UV laser) and single cell (with 2-photon laser) activation. Fish were imaged at 2dpf. Scale bar: 200 μm. Data are presented as mean ± SEM. ***p < 0.001; N.S, not significant. |
|
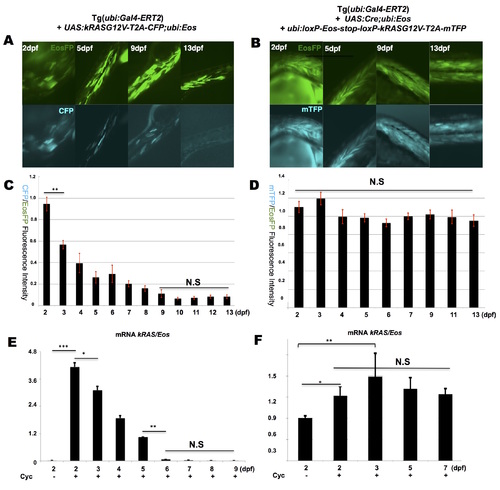
Dynamic profile of kRASG12V expression over time following transient and constitutive cyclofen activation. Transient kRASG12V induction was achieved by injecting UAS:kRASG12V-T2A-CFP; ubi:Eos plasmid into Tg(ubi:Gal4-ERT2) embryos followed by a transient incubation in cyclofen. Constitutive induction was achieved by co-injecting UAS:Cre; ubi:Eos and ubi:loxP-Eos-loxP-kRASG12V-T2A-mTFP plasmids into Tg(ubi:Gal4-ERT2) embryos followed by a transient incubation in cyclofen. (A, B) The fluorescent expression of both EosFP and CFP (or mTFP) co-expressed with kRASG12V was imaged over 13 days. Quantitative measurements of the fluorescent intensity and qPCR analysis (of kRASG12V transcripts) displayed a gradual decrease of kRASG12V expression upon transient induction (C, E), and a stable kRASG12V expression upon constitutive induction (D, F). Scale bar: 400 μm. Data are presented as mean ± SEM. ***p < 0.001; **p < 0.01; *p < 0.05; N.S, not significant. |
|
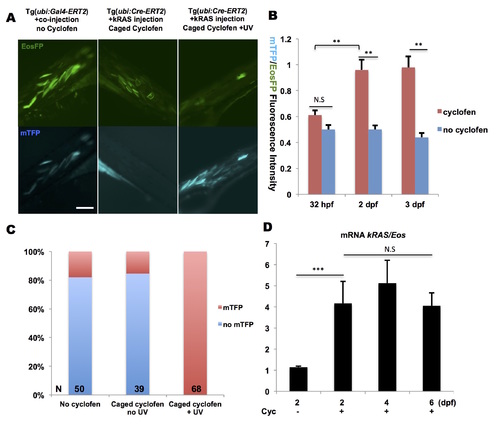
Optical control of constitutive expression by Cre-recombinase. (A, left panel) Tg(ubi:Gal4-ERT2) embryos were injected at the one-cell stage with UAS:Cre; ubi:Eos and ubi:loxP-Eos-loxP-kRAS-T2A-mTFP plasmids. At 32 hpf, embryos displayed expression of mTFP in spite of absence of cyclofen, indicative of residual activity of Cre-recombinase possibly due to leakage of the UAS promoter. (B) Upon cyclofen treatment at 32 hpf, the expression of mTFP at 2dpf and 3dpf was higher than the control (without cyclofen) . (A, middle panel) Injection of ubi:loxP-Eos-stop-loxP-kRASG12V-T2A-mTFP plasmid into Tg(ubi:Cre-ERT2) embryos display no leakage in most of the non-activated embryos, as evidenced by the absence of mTFP expression following incubation in caged cyclofen without UV uncaging (despite autofluorescence from the yolk). (A, right panel and C) On the other hand, upon UV illumination and release of cyclofen, the Eos sequence was effectively excised (resulting in lower fluorescence in the EosFP channel) and the mTFP expression (and fluorescence) was turned on. (D) With Tg(ubi:Cre-ERT2) embryos injected with ubi:loxP-Eos-stop-loxP-kRASG12V-T2A-mTFP plasmid, RT-qPCR analysis of Eos and kRASG12V mRNA showed significant increase of kRAS/Eos ratio upon cyclofen activation. Scale bar: 200 μm. Data are presented as mean ± SEM. **p < 0.01; ***p < 0.001; N.S, not significant. |
|
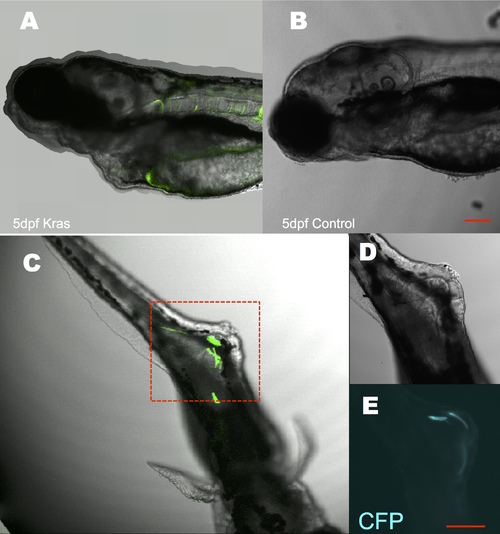
Transient induction of kRASG12V at an early stage resulted in developmental and tumorigenic defects. An early administration of cyclofen at the one-cell stage caused severe developmental defects in 5 dpf fish (A) compared to non-treated control (B). Many fish also exhibited tumorigenic and hyperplasic tissues (C) with remaining CFP expression (D, E zoom-in images of C). Scale bar: 200 μm. |
|
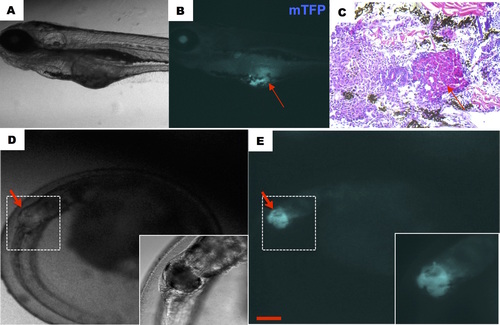
Early tumorigenesis caused by constitutive activation of kRASG12V. Constitutive activation was induced at 1dpf and representative fish showed early tumorigenesis at 5dpf (A-C) and 3dpf (D, E). Notice the hyperplasic tissue (shown by an arrow in B, C), which displayed upon H&E staining a pack of cells with condensed nuclei and an increased nuclei/cytoplasm ratio characteristic of tumors. Scale bar: 200 μm. |
|
Representative tumors induced by constitutive kRASG12V activation. Tg(ubi:Cre- ERT2) fish injected with ubi:loxP-Eos-stop-loxP-kRASG12V-T2A-mTFP plasmid were incubated at 1dpf in cyclofen or caged cyclofen + UV. Various tumors (noted by arrows) were observed in fish at 2 (A), 6 (E) and 12 (I) months. The tumors expressed reduced EosFP (B, F, J), and were characterized by both strong expression of mTFP (C, G, K) and histopathological analysis (D, H, L). Condensed nuclei and distorted cell shapes are typical of tumor morphologies, however the different overall textures in D, H and L suggest different tumor types. Scale bar: B, D, F, G, J, 400 μm; D, H 200 μm; L 50 μm. |
|
Local transient activation of oncogene expression with a UV laser at different stages and in various tissues. We crossed a stable Tg(ubi:Eos;UAS:kRASG12V-T2A-CFP) line with a Tg(ubi:Gal4-ERT2) line. Embryos were pre-incubated in 4 μM caged cyclofen for 4 hours. They were illuminated at 10 hpf (A) or 32 hpf (B, C) with the 405 nm line of a LEICA SP5-BLUE microscope for 5 seconds. Fluorescent images were taken after 18 hours and showed precise spatial control of oncogene activation. While all locally illuminated embryos developed normally, many globally illuminated fish (D) exhibited severe developmental defects and died. Scale bar: 400 μm. |
|
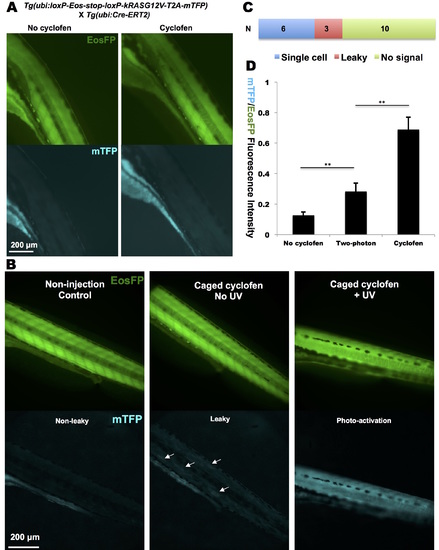
Constitutive activation of kRASG12V in transgenic fish lines. (A) Cyclofen was not able to induce mTFP expression in the heterozygous Tg(ubi:loxP-Eos-stop-kRASG12V-T2AmTFP; ubi:Cre-ERT2) embryo. (B) Oncogene activation could be observed in a Tg(ubi:loxPEos- stop-kRASG12V-T2A-mTFP) injected with Cre-ERT2 mRNA: distinct mTFP expression patterns in non-activated, leaky (see arrows) and photo-activated embryos are shown. (C) Success rate of two-photon activation of the oncogene (via the fluorescence of the reporter protein, mTFP) in 19 embryos. (D) Quantification of two-photon activated kRASG12V signal compared to non-activated and cyclofen-activated mTFP/EosFP fluorescent ratios. Data are presented as mean ± SEM. **p < 0.01. |
|
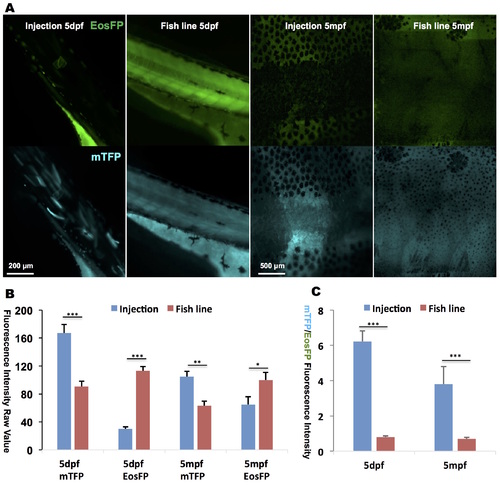
Lower expression levels of mTFP are induced in the transgenic line as compared to the injected fish. (A) Global photoactivation with a UV lamp of caged cyclofen resulted in higher mTFP fluorescence (and lower Eos fluorescence) in the injected fish than in the transgenic line at both 5 dpf and 5 mpf, measured by both mTFP and EosFP raw intensity (B) and mTFP/EosFP ratio (C). Data are presented as mean ± SEM. **p < 0.01; ***p < 0.001. |