- Title
-
Cyp1b1 Regulates Ocular Fissure Closure Through a Retinoic Acid-Independent Pathway
- Authors
- Williams, A.L., Eason, J., Chawla, B., Bohnsack, B.L.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
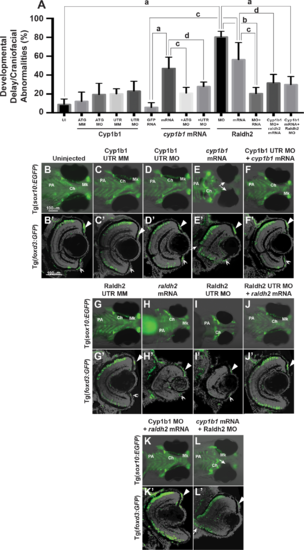
Overexpression of cyp1b1 inhibited ocular fissure closure via an RA-independent pathway. The percentage of 72 hpf embryos with developmental delay and/or craniofacial abnormalities (A) due to Cyp1b1 MO (ATG or 5′ UTR) knockdown, cyp1b1 (mRNA) overexpression, Raldh2 MO knockdown, raldh2 (mRNA) overexpression compared to uninjected, mismatch (MM) MO-injected or gfp mRNA-injected controls. ([a] P < 0.0001; [b] P < 0.001; [c] P < 0.01; [d] P < 0.05). Live images of uninjected (B) and mismatch control-injected (C, G) Tg(sox10:EGFP) embryos showed that at 96 hpf, neural crest contributed to the pharyngeal arches (PA) and the ceratohyal (Ch) and Meckel's (Mk) cartilage of the jaw. Sections of 96 hpf Tg(foxd3:GFP) embryos showed that foxd3-positive neural crest cells contributed to the iris stroma in the dorsal (B', C', G', arrowhead) and ventral (double arrowhead) iridocorneal angle. In addition, differentiated retinal photoreceptors expressed foxd3, which was unrelated to neural crest cells. Injection of 5′ UTR Cyp1b1 MO had minimal effect on craniofacial neural crest (D) and overall eye (D') development. Overexpression of cyp1b1 through mRNA injection disrupted neural crest–derived Meckel's cartilage formation (E, K) and inhibited ocular fissure closure, resulting in prominent colobomas (closed arrows, E, E'). The retinal layers were properly differentiated, including the expression of foxd3. Injection of Cyp1b1 5′ UTR MO improved the ocular and neural crest phenotypes due to cyp1b1 mRNA (F, F'). As previously demonstrated, increasing (raldh2 mRNA, H, H') or decreasing (Raldh2 5′ UTR MO, I, I') RA inhibited pharyngeal arch development, disrupted ceratohyal and Meckel's cartilage formation, and decreased eye size. Injection of raldh2 mRNA rescued the ocular and neural crest phenotypes induced through Raldh2 5′ UTR MO knockdown (J, J'). Cyp1b1 MO improved eye size, retinal differentiation, and neural crest–derived pharyngeal arch and ceratohyal and Meckel's cartilage formation in embryos injected with raldh2 mRNA (K, K'). Knockdown of Raldh2 improved neural crest–derived pharyngeal arch and ceratohyal and Meckel's cartilage formation (L'), but did not rescue ocular fissure closure (arrows, L') in embryos injected with cyp1b1 mRNA. |
|
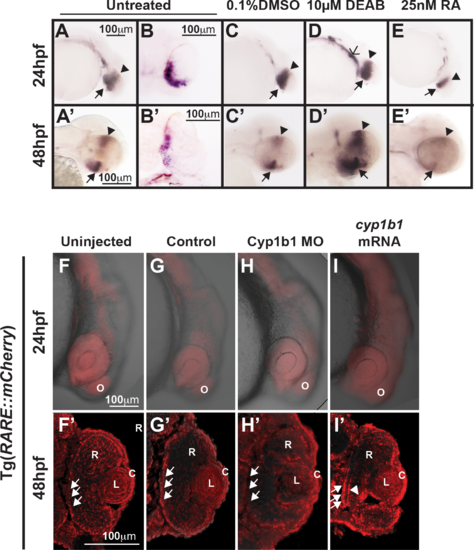
Cyp1b1 expression in the developing eye was regulated through RA. Whole-mount in situ hybridization showed cyp1b1 expression along the dorsal (arrowheads)–ventral (arrows) axis in the developing eye at 24 and 48 hpf in uninjected (A, A') and 0.1% DMSO-treated (C, C') embryos. In sections, cyp1b1 localized to the retina and retinal pigment epithelium (B, B'). Treatment with 10 μM DEAB at 12 hpf increased cyp1b1 expression in the ventral neural crest, which subsequently forms the brachial arches (D, open arrowhead) at 24 hpf and the dorsal (arrowheads) and ventral (arrows) eye at 48 hpf (D'). Exogenous treatment with 25 nM all-trans RA at 12 hpf decreased cyp1b1 expression in the dorsal and ventral eye at 24 (E) and 48 hpf (E'). Live imaging of the Tg(RARE:mCherry) reporter line showed areas of high RA activity in the eye and olfactory placode (O) in uninjected (F) and control-injected (G) embryos. Within the eye (F', G'), RA activity was observed in the retinal pigment epithelium (arrows), retina (R), lens (L), and cornea (C). MO knockdown of Cyp1b1 (H, H') showed decreased RA activity in the medial retinal pigment epithelium (arrows) correlated with the area of cyp1b1 expression (B'). Overexpression of cyp1b1 through mRNA injection diffusely increased mCherry expression at 24 hpf throughout the craniofacial region (I). Increased RA activity in the eye was also observed in the medial retinal pigment epithelium (arrows) and ocular fissure (arrowhead). |
|
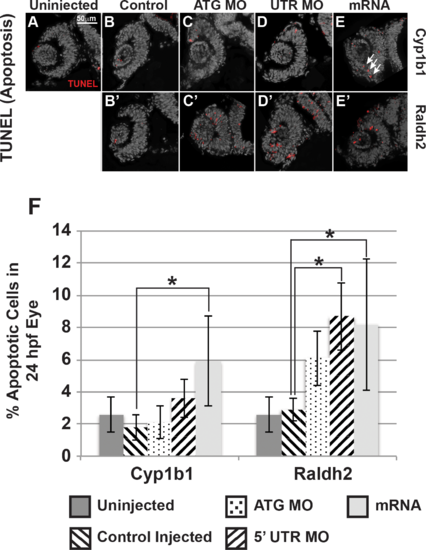
Cyp1b1 and RA regulated cell survival in the developing eye. Cyp1b1 ATG (C) and 5′ UTR (E) MO knockdown did not significantly affect apoptosis in the eye at 24 hpf compared to uninjected (A) and control-injected (B) embryos. Cyp1b1 overexpression (E) increased apoptosis and the apoptotic cells were specifically localized to the inferior ocular fissure (arrows). Raldh2 5′ UTR (D') but not ATG (C') MO knockdown significantly decreased cell survival throughout the developing eye compared to uninjected (A) and control injected (B'). Similarly, overexpression of raldh2 (E') increased apoptosis in the eye. The percentage of apoptotic cells in 24 hpf developing eyes (F) of embryos injected with Cyp1b1 MO (ATG or 5′ UTR), cyp1b1 mRNA, Raldh2 (ATG or 5′ UTR) MO, raldh2 mRNA compared to uninjected and control injected (*P < 0.01). PHENOTYPE:
|
|
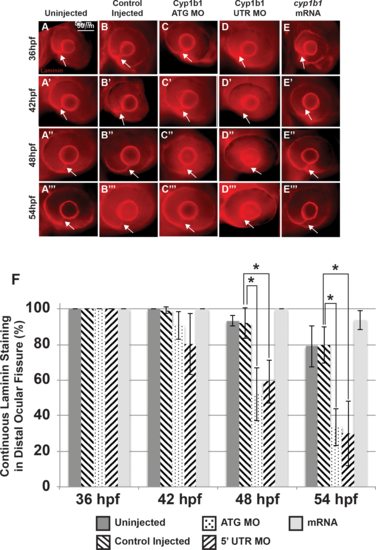
Alterations in the basement membrane integrity as a result of decreased or increased Cyp1b1. Whole-mount laminin staining showed that in uninjected (A–A''') and control-injected (B–B''') embryos, a continuous basement membrane (arrows) was evident in the distal ocular fissure at 36, 42, 48, and 54 hpf. Cyp1b1 ATG (C–C''') and 5′ UTR (D–D''') MO knockdown showed discontinuous staining of laminin at 48 (C'', D'') and 54 hpf (C''', D'''), suggesting premature basement membrane breakdown. In cyp1b1 mRNA-injected embryos, laminin staining remained prominent within the fissure at all time points (E–E'''). The percentage of embryos with continuous laminin staining in the distal ocular fissure at 36, 42, 48, and 54 hpf (G), (*P < 0.001). |
|
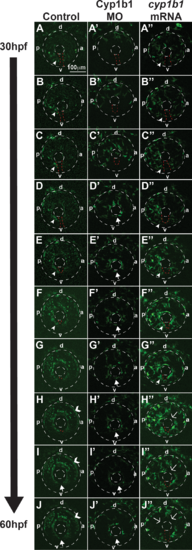
Cyp1b1 regulates ocular neural crest migration. Time-lapse imaging of Tg(foxd3:EGFP) control embryos from 30 to 60 hpf (A–J) showed that foxd3-positive cells entered into the anterior chamber between the surface ectoderm and optic cup, with more cells localized to the dorsal (d)–posterior (p) quadrant (E–H) compared with the anterior (a) and ventral (v) areas. In addition, foxd3-positive cells migrated adjacent to and through the ocular fissure (A–F, white arrowhead denotes migrating neural crest, red dashed line demarcates ocular fissure). At 60 hpf, foxd3-positive cells completely encircled the lens (I, J, closed arrows), indicating the closure of the fissure. In addition, at 60 hpf, foxd3 was also expressed in photoreceptors (H–J, open arrowhead). In Cyp1b1 MO knockdown embryos, Foxd3-positive cells travelled between the surface ectoderm and optic cup in the dorsal quadrants (A'–E'), but there were few cells adjacent to and in the ocular fissure. Foxd3-positive cells encircled the lens prior to controls (D'–H' versus D–H), indicating the premature closure of the fissure. At 60 hpf, there were fewer foxd3-positive cells in the eye (J'). In embryos overexpressing cyp1b1, ocular neural crest migration was less organized, as foxd3-positive cells were located throughout the posterior half of the eye (A''–G''). Further, the ocular fissure remained open and prevented the continuous coalescence of neural crest cells around the lens (H''–J'', open arrows), resulting in fewer neural crest cells at 60 hpf (J''). |
|
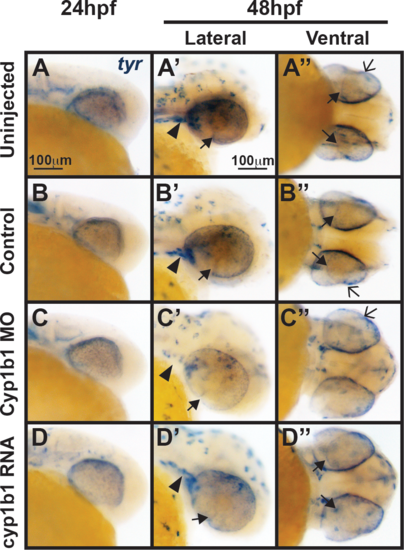
Tyr expression is not influenced by alterations in cyp1b1 expression. In situ hybridization demonstrated that at 24 hpf, tyr was expressed in the migrating neural crest and retinal pigment epithelium in uninjected (A) and control-injected (B) embryos. At 48 hpf, tyr expression (A', A'', B', B'') followed the skin pigmentation pattern and was also observed in the cranial neural crest (arrowheads), retinal pigment epithelium, migrating neural crest cells in the ocular fissure (closed arrows), and iris stroma (open arrows). Knockdown of cyp1b1 did not alter tyr expression at 24 hpf, but did decrease tyr expression in the cranial neural crest (arrowhead) and ocular fissure (C, C') at 48 hpf. Overexpression of cyp1b1 did not alter tyr expression (D, D', D'') at 24 and 48 hpf. |
|
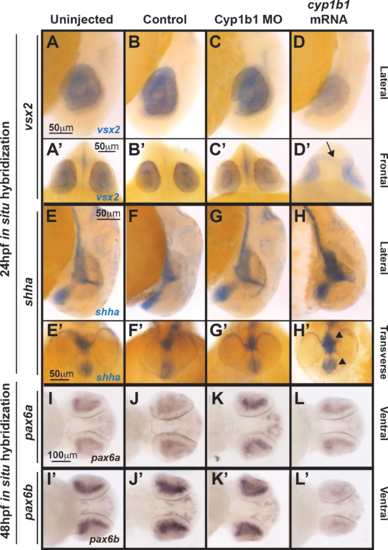
Cyp1b1 differentially regulated vsx2, shh, and pax6. Whole-mount in situ hybridization at 24 hpf demonstrated that vsx2 expression in the eye decreased in response to cyp1b1 overexpression (D, D') compared with Cyp1b1 MO knockdown (C, C'), control-injected (B, B'), and uninjected (A, A'') embryos. Note the appearance of tissue bridging the two optic cups in the cyp1b1 mRNA-injected embryo, as demarcated by vsx2 (arrow). Shha at 24 hpf showed increased expression in the midline floor plate between the eyes in embryos injected with cyp1b1 mRNA (arrowheads, H, H') compared with Cyp1b1 MO knockdown (G, G'), control -injected (F, F'), and uninjected (E, D') embryos. Pax6a and pax6b expression in the retina at 48 hpf decreased in response to cypb1 overexpression (L, L') compared with Cyp1b1 knockdown (K, K'), control-injected (J, J'), and uninjected (I, I') embryos. |
|
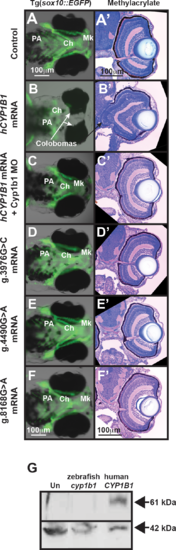
Human CYP1B1 and zebrafish cyp1b1 genes are evolutionarily and functionally conserved. Live images and methylacrylate sections of 96 hpf Tg(sox10:EGFP) embryos demonstrated that the injection of human CYP1B1 mRNA disrupted neural crest–derived jaw and pharyngeal arch formation and inhibited ocular fissure closure (B, B') compared with controls (A, A'). The knockdown of endogenous Cyp1b1 restored fissure closure (C') and partially rescued the jaw and pharyngeal arch defects resulting from the injection of human CYP1B1 mRNA (C). The injection of human CYP1B1 mRNA containing clinically relevant g.3976G>C (D, D'), g.4490G>A (E, E'), or g.8168G>A (F, F') mutations did not disrupt eye or neural crest development. Western blot analysis (G) showed expression of human CYP1B1 protein (61 kDa) in embryos injected with human CYP1B1 mRNA sequence, but not in uninjected embryos or embryos injected with zebrafish cyp1b1 mRNA. β-actin (42 kDa) was used as a loading control. EXPRESSION / LABELING:
|