- Title
-
Myomaker is required for the fusion of fast-twitch myocytes in the zebrafish embryo
- Authors
- Zhang, W., Roy, S.
- Source
- Full text @ Dev. Biol.
|
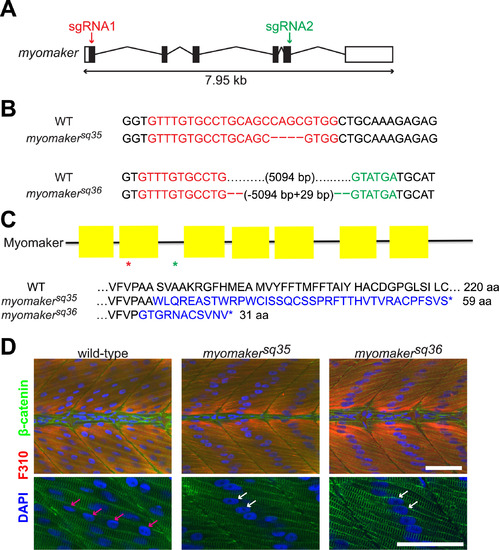
Myomaker mutants are defective in myocyte fusion. A: Schematic diagram of myomaker gene structure. White and black boxes represent UTRs and exons respectively, and the lines indicate introns. The arrows indicate regions targeted by sgRNAs. B: Sequences of wild-type and myomaker alleles showing the mutations. Deleted bases are shown as dashes, while sgRNA1 and sgRNA2 target regions are shown in red and green respectively. C: Diagram of Myomaker protein structure and predicted protein sequences of myomaker alleles. The yellow boxes in the diagram represent the transmembrane domains, while the red and green asterisks indicate the premature STOP codon positions of the myomakersq36 and myomakersq35 mutant proteins respectively. For the protein sequences, the sequences in blue are those that are different from wild-type sequence. D: Fast-twitch muscle cells of 2 d.p.f. myomaker mutant embryos are mostly mononucleated, unlike the multinucleated fast-twitch muscle cells of wild-type embryos. Fast-twitch muscle cells are labeled with F310 antibody, with the nuclei and cell membranes stained with DAPI and anti-β-catenin antibodies, respectively. The bottom panel shows magnified images of a few muscle cells from the top panel. The pink arrows indicate the multiple nuclei within a wild-type muscle cell, while the white arrows indicate the single nuclei of mononucleated mutant muscle cells. Scale bar=50 µm. |
|
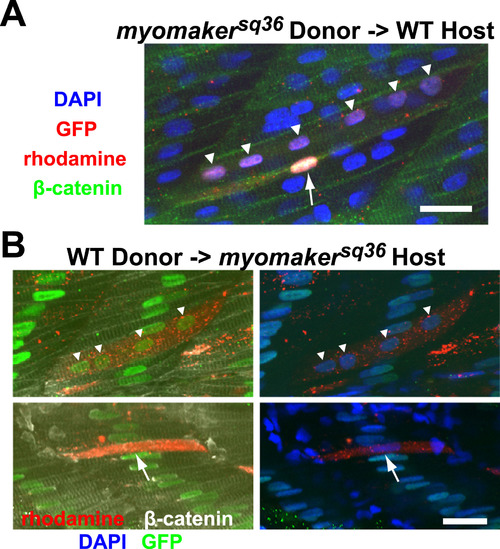
Myomaker is not required cell autonomously for myocyte fusion. A: A portion of donor myomakersq36 mutant cells transplanted into wild-type host can undergo myocyte fusion. The image shows a rhodamine-labeled donor mutant cell, which also expresses H2B-GFP in its nuclei, that underwent fusion to form a multinucleated cell with 6 nuclei (indicated by triangles), and a rhodamine-labeled donor mutant cell that did not undergo fusion and remained mononucleated (indicated by an arrow). All nuclei and cell membranes are stained with DAPI and anti-β-catenin antibodies respectively, while H2B-GFP is labeled with anti-GFP staining. The image is a projection of 2 Z-sections. Scale bar=20 µm. B: Some donor wild-type cells are able to fuse with myomakersq36mutant cells when transplanted into the myomakersq36 mutant host. Top panel shows a rhodamine-labeled multinucleated chimeric cell expressing H2B-GFP in all its nuclei (indicated by triangles). The chimeric cell resulted from fusion between rhodamine-labeled donor wild-type cell and host mutant cells that express H2B-GFP in the nuclei. The bottom panel shows a rhodamine-labeled donor wild-type cell that did not undergo fusion and remained mononucleated (indicated by an arrow). Nuclei and cell membranes are stained with DAPI and anti-β-catenin antibodies respectively, while H2B-GFP is labeled with anti-GFP staining. The top and bottom panel images are projections of 5 and 3 Z-sections respectively. Scale bar=20 µm. |
|
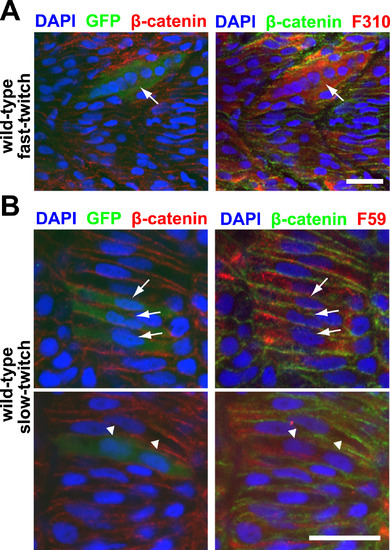
Myomaker overexpression is sufficient to drive ectopic fusion in both fast-twitch and slow-twitch muscle cells. A: A 36 h.p.f. embryo with a Myomaker-expressing (as reported by anti-GFP staining) fast-twitch muscle cell displaying hyperfusion (arrow). Fast-twitch muscle cells are labeled with F310 staining, and nuclei and cell membranes are labeled with DAPI and anti-β-catenin staining respectively. Scale bar=20 µm. B: A small percentage of Myomaker-expressing slow-twitch muscle cells can fuse. Top panel shows 3 mononucleated Myomaker-expressing slow-twitch cells (indicated by arrows), while bottom panel shows a binucleated Myomaker-expressing slow-twitch cell (indicated by triangles). The embryos are 21 h.p.f. and stained with anti-GFP (to report Myomaker expression) and F59 antibody (to detect slow-twitch muscle cells). Nuclei and cell membranes are labeled with DAPI and anti-β-catenin staining respectively. Scale bar=20 µm. |
|
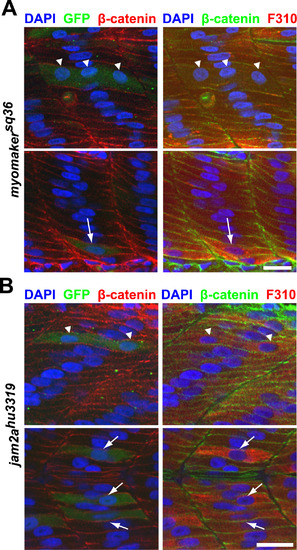
Myomaker overexpression is sufficient to partially rescue myocyte fusion in myomakersq36 and jam2ahu3319 mutants. A: Myomaker-expressing cells (as reported by anti-GFP staining) in myomakersq36mutant embryos can undergo fusion to form multinucleated fast-twitch muscle cells (Top panel; triangles indicate 3 nuclei within a Myomaker-expressing cell), or remain unfused (Bottom panel; arrow indicates a Myomaker-expressing mononucleated cell). Embryos are 36 h.p.f. and stained with DAPI, anti-β-catenin and F310 antibodies to label nuclei, cell membranes and fast-twitch muscle cells, respectively. Scale bar=20 µm. B: Myomaker overexpression can partially rescue myocyte fusion in jam2ahu3319 mutants. Top panel depicts a binucleated Myomaker-expressing (reported by anti-GFP staining) fast-twitch muscle cell (nuclei indicated by triangles), and the bottom panel shows 3 mononucleated Myomaker-expressing fast-twitch muscle cells (indicated by arrows). All embryos are 36 h.p.f. jam2ahu3319 mutants that have also been stained with DAPI, anti-β-catenin and F310 antibodies to label nuclei, cell membranes and fast-twitch muscle cells, respectively. Scale bar=20 µm. |
|
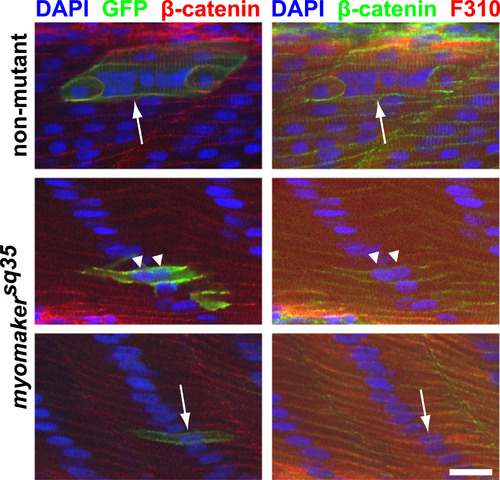
caRac is unable to induce robust ectopic fusion in myomakersq35 mutants. caRac can induce hyperfusion in non-mutant embryos (Top panel; arrow indicates a hyperfused caRac-expressing cell (labeled by anti-GFP staining)), but not in myomakersq35 mutants. Most caRac-expressing fast-twitch muscle cells in myomakersq35mutants are mononucleated (Bottom panel; arrow indicates an unfused cell), and a few cells are binucleated (Middle panel; triangles indicate the nuclei of a binucleated cell). All embryos are 2 d.p.f. and also stained with DAPI, anti-β-catenin and F310 antibodies to label nuclei, cell membranes and fast-twitch muscle cells, respectively. The bottom panel images are projections of 2 Z-sections. Scale bar=20 µm. |
|
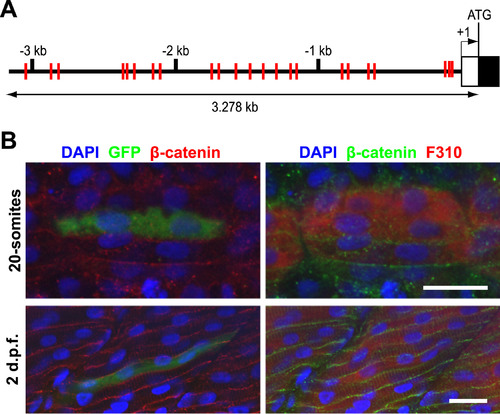
A myomaker promoter fragment with multiple E-boxes is sufficient to direct reporter expression in the fast-twitch muscle lineage. A: Schematic diagram of 5′ genomic region of myomaker. The black and white boxes represent Exon 1 and 5′ UTR respectively, while the red lines indicate locations of the 23 E-box sequences. B: The 3.278 kb promoter fragment is sufficient to drive GFP expression (detected with anti-GFP staining) in fast-twitch muscle cells (labeled with F310 antibody), as observed in both 20-somites (Top panel) and 2 d.p.f. embryos (Bottom panel). Nuclei and cell membranes are identified by DAPI and anti-β-catenin antibodies. Scale bar=20 µm. |
Reprinted from Developmental Biology, 423(1), Zhang, W., Roy, S., Myomaker is required for the fusion of fast-twitch myocytes in the zebrafish embryo, 24-33, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.