- Title
-
Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity.
- Authors
- Li, J., Casteels, T., Frogne, T., Ingvorsen, C., Honoré, C., Courtney, M., Huber, K.V., Schmitner, N., Kimmel, R.A., Romanov, R.A., Sturtzel, C., Lardeau, C.H., Klughammer, J., Farlik, M., Sdelci, S., Vieira, A., Avolio, F., Briand, F., Baburin, I., Májek, P., Pauler, F.M., Penz, T., Stukalov, A., Gridling, M., Parapatics, K., Barbieux, C., Berishvili, E., Spittler, A., Colinge, J., Bennett, K.L., Hering, S., Sulpice, T., Bock, C., Distel, M., Harkany, T., Meyer, D., Superti-Furga, G., Collombat, P., Hecksher-Sørensen, J., Kubicek, S.
- Source
- Full text @ Cell
|
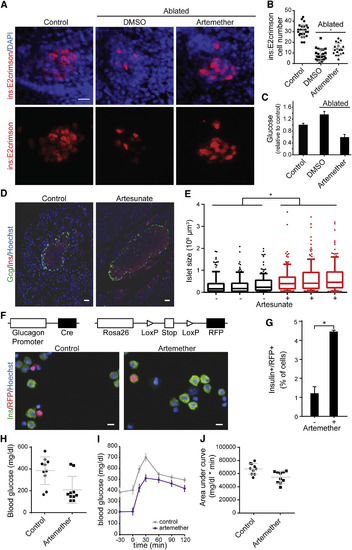
Artemether Increases β Cell Mass In Vivo (A) Representative images of insulin reporter cells in zebrafish larvae. β cells in ins:caspase8;ins:E2Crimson embryos were ablated by treatment with 2 μM dimerizer AP20187 from 3 dpf to 5 dpf. Then, larvae were treated with 5 μM artemether or control DMSO for 4 days (8–12 dpf) and immunostained for dsRed and DAPI. Scale bar, 15 μm. (B) Quantification of numbers of insulin reporter cells in zebrafish larvae described in (A). Shown are cell numbers from individual animals, and the means and SD are indicated for the three groups. ∗p = 0.0005. (C) Glucose measurement in pooled larvae extracts, treated as in (A) and normalized to non-ablated animals (means and SD are indicated for at least four independent larvae pools for each condition). (D) Representative staining for insulin and glucagon in mouse pancreas following a 3-month treatment with 1 mg/mL artesunate in drinking water versus control DMSO. Scale bar, 30 μm. (E) Quantification of islets size in sections from mouse pancreata following a 3-month treatment with artesunate in drinking water (120–180 islets per animal). ∗p < 0.001. Shown are medians, 25%–75% (boxes) and 10%–90% confidence intervals. (F) Co-staining of RFP and insulin in RFP-labeled lineage-tracing mouse islets after treatment with 10 μM artemether or control DMSO for 24 hr. Scale bar, 10 μm. (G) Quantification of RFP/insulin double-positive cells in mouse islet from (F) (means and SD are indicated from two replicates). ∗p = 0.019. (H) Blood glucose levels after an overnight fast in a rat β cell ablation model. β cells were ablated with 60 mg/kg streptozotocin; on day 9 post-ablation, animals were assigned to treatment and control groups based on matching fasting glucose levels and were treated with 20 mg/kg artemether p.o. for 7 days followed by 200 mg/kg artemether p.o. for 16 days. (Measured values in individual animals, means and SD, n = 10 per group, p = 0.005). (I) Oral glucose tolerance test of animals described in (H). Means and SE are indicated for ten animals from each group. (J) Area under the curve from the oral glucose tolerance test described in (I). Shown are measurements in individual animals, and the means and SD are indicated for the groups; p = 0.004. See alsoFigure S5. |
|
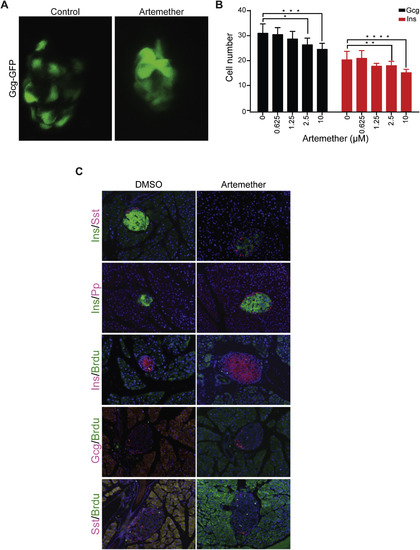
Effect of Artemether on Pancreatic Islets In Vivo, Related to Figure 5 (A) Islet morphology and GFP expression as marker of α cells of Tg(Gcga:GFP)ia1 zebrafish larvae treated from 26 hpf until 100 hpf with artemether. (B) Quantification of Glucagon-GFP positive cells and Insulin-mCherry positive cells per single zebrafish islets (8-10 fish per condition; ∗p = 0.007, ∗∗p = 0.088, ∗∗∗p = 0.004, ∗∗∗∗p = 0.003). Error bars represent mean ± SD. (C) Immunofluorescence for Sst, Pp, and BrdU co-stained with insulin or glucagon in mouse pancreas with or without artemether treatment. |
Reprinted from Cell, 168(1-2), Li, J., Casteels, T., Frogne, T., Ingvorsen, C., Honoré, C., Courtney, M., Huber, K.V., Schmitner, N., Kimmel, R.A., Romanov, R.A., Sturtzel, C., Lardeau, C.H., Klughammer, J., Farlik, M., Sdelci, S., Vieira, A., Avolio, F., Briand, F., Baburin, I., Májek, P., Pauler, F.M., Penz, T., Stukalov, A., Gridling, M., Parapatics, K., Barbieux, C., Berishvili, E., Spittler, A., Colinge, J., Bennett, K.L., Hering, S., Sulpice, T., Bock, C., Distel, M., Harkany, T., Meyer, D., Superti-Furga, G., Collombat, P., Hecksher-Sørensen, J., Kubicek, S., Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity., 86-100.e15, Copyright (2017) with permission from Elsevier. Full text @ Cell