- Title
-
Knockout of Zebrafish Ovarian Aromatase Gene (cyp19a1a) by TALEN and CRISPR/Cas9 Leads to All-male Offspring Due to Failed Ovarian Differentiation
- Authors
- Lau, E.S., Zhang, Z., Qin, M., Ge, W.
- Source
- Full text @ Sci. Rep.
|
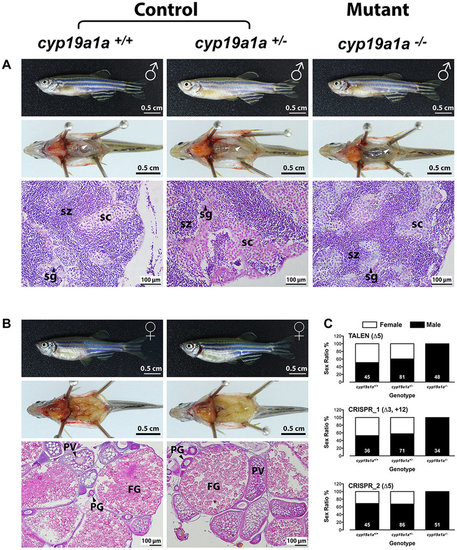
All-male development in cyp19a1a mutants. (A and B) Anatomical and histological examination of the gonads in the control (cyp19a1a+/+ and cyp19a1a+/−) and mutant (cyp19a1a−/−). (C) Sex ratio of the three cyp19a1a mutants. The number in each column indicates the sample size. sg, spermatogonia; sc, spermatocytes; sz, spermatozoa; PG, primary growth follicle; PV, previtellogenic follicle; FG, full-grown follicle. PHENOTYPE:
|
|
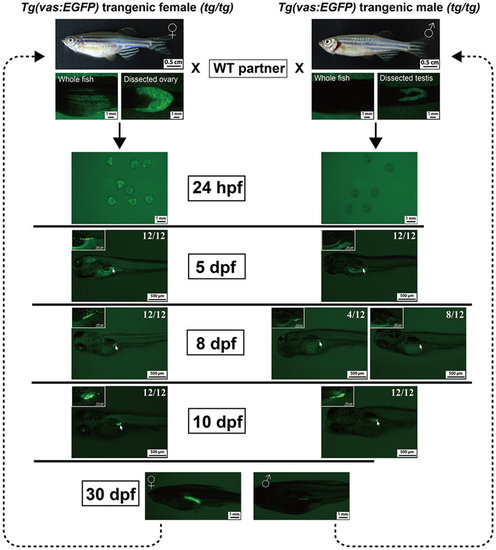
Expression of GFP in the gonads of the transgenic line Tg(vas:EGFP). The embryos/offspring were obtained by crossing the WT fish with either female (tg/tg) or male (tg/tg) transgenic fish. The GFP signal was observed under fluorescent microscope from 24 hpf to 30 dpf (12 individuals each time). EXPRESSION / LABELING:
|
|
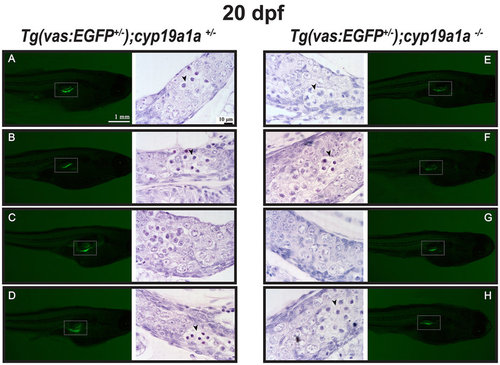
Gonad development at 20 dpf in the control [Tg(vas:EGFP);cyp19a1a+/−; fish A–D] and mutant [Tg(vas:EGFP);cyp19a1a−/−; fish E–H]. Similar GFP signals (boxed in the photo) were observed in the two groups and histological examination showed no significant difference. Meiotic germ cells (arrowhead) with condensed chromatin were often seen in both the mutant and control gonads. EXPRESSION / LABELING:
PHENOTYPE:
|
|
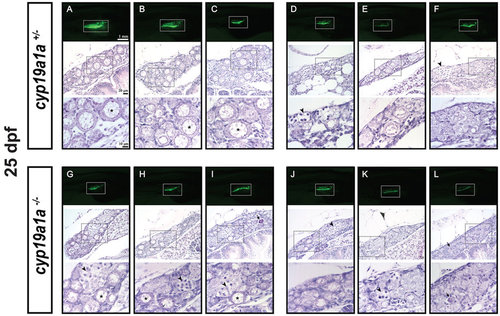
Gonad development at 25 dpf in the control (cyp19a1a+/−; fish A–F) and mutant (cyp19a1a−/−; fish G–L). The GFP intensity in the gonads (boxed in the photo) started to diverge in the control (A–C vs. D–F) but not the mutant fish (G–L), suggesting the start of gonadal differentiation. Two groups of fish could be distinguished by histological analysis. One group had well-developed EPOs (asterisks) in the gonads (fish A–C) in the control and (G–I) in the mutant) and the other contained mostly undifferentiated germ cells (fish D–F) in the control and (J–L) in the mutant. The EPOs in the control fish (A–C) were generally larger with stronger GFP signal than those in the mutant fish (G–I). Numerous meiotic germ cells (arrowheads) were present in the gonads of both control and mutant fish. The dark-stained condensed apoptotic germ cells (arrows) were often observed at this stage, indicating the process of juvenile ovary-testis transformation. PHENOTYPE:
|
|
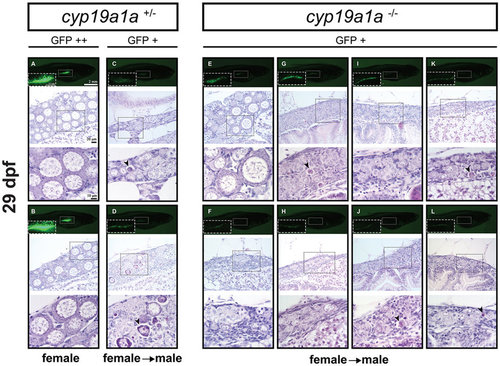
Gonad development at 29 dpf in the control (cyp19a1a+/−; fish A–D) and mutant (cyp19a1a−/−; fish E–L). In the control fish, the gonads (boxed in the photo) with much enhanced GFP signal (GFP++; A and B) contained large LPOs and little amount of stromal tissues, indicating the development towards the true ovary whereas those with weak or reduced GFP signal (GFP+; C and D) had few POs but large number of apoptotic cells (arrowheads), suggesting the development towards males. In the mutant fish, all the mutant individuals showed weak GFP signal comparable with that of the control males. Most mutant gonads (G–L) contained undifferentiated germ cells with some undergoing apoptosis (arrowheads). A few contained OPs (e.g., fish E) but with large amount of stromal tissues. PHENOTYPE:
|
|
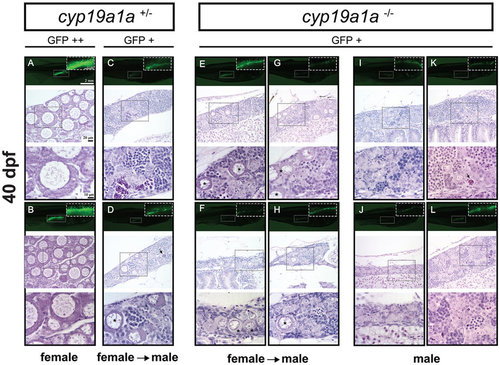
Gonad development at 40 dpf in the control (cyp19a1a+/−; fish A–D) and mutant (cyp19a1a−/−; fish E–L). The control fish had well-differentiated ovary (A and B) and testis (C and D), whereas all the mutant individuals were undergoing or had completed ovary-testis transformation with typical testicular tissues containing different stages of spermatogenic cells. Some individuals (E–H) still contained a few typical EPOs (asterisks) scattered among the testicular tissues. Arrows indicate the apoptotic germ cells. PHENOTYPE:
|
|
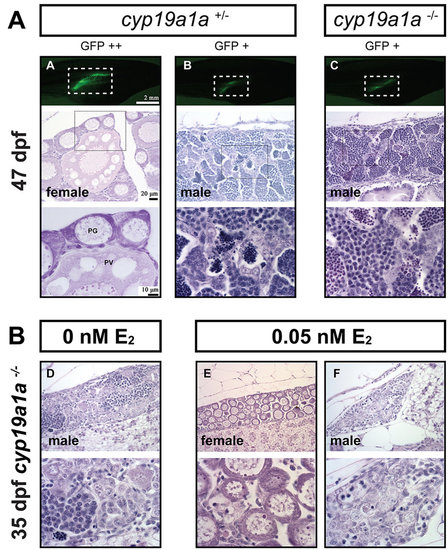
(A) Gonad development at 47 dpf in the control (cyp19a1a+/−) and mutant (cyp19a1a−/−). The gonadal differentiation had finished in the control fish with the ovary and testis well developed. The ovary contained both PG and PV follicles, indicating puberty onset in the female. All mutant fish were males with well-developed testes containing all stages of spermatogenic cells without any oocytes. (B) Rescue of mutant phenotype by E2 treatment. The juvenile mutant fish were treated with E2 (0.05 nM) from 15 to 30 dpf, and the fish were sampled for histological examination at 35 dpf. E2 treatment could induce normal ovarian formation in some mutant fish as shown by fish E. Other fish (e.g., fish F) still had undifferentiated gonads that were likely destined to males. PHENOTYPE:
|
|
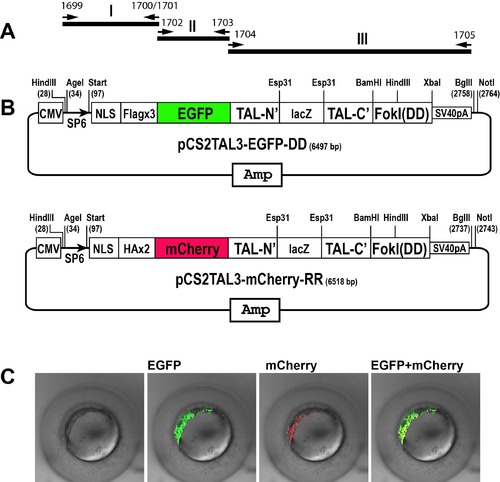
Schematic representation of the EGFP and mCherry-tagged TALEN plasmids. A) The assembling strategy to insert EGFP and mCherry genes into the TALEN backbone plasmids. B) Structure of pCS2TAL3-EGFP-DD and pCS2TAL3- mCherry-RR. C). Embryos injected with in vitro transcribed mRNA of cyp19a1a-pCS2TAL3- EGFP-DD and cyp19a1a-pCS2TAL3-mCherry-RR. Both TALE proteins were translated and the yellow signal indicates co-translation of the proteins in the same cells. |