- Title
-
Zebrafish P54 RNA helicases are cytoplasmic granule residents that are required for development and stress resilience
- Authors
- Zampedri, C., Tinoco-Cuellar, M., Carrillo-Rosas, S., Diaz-Tellez, A., Ramos-Balderas, J.L., Pelegri, F., Maldonado, E.
- Source
- Full text @ Biol. Open
|
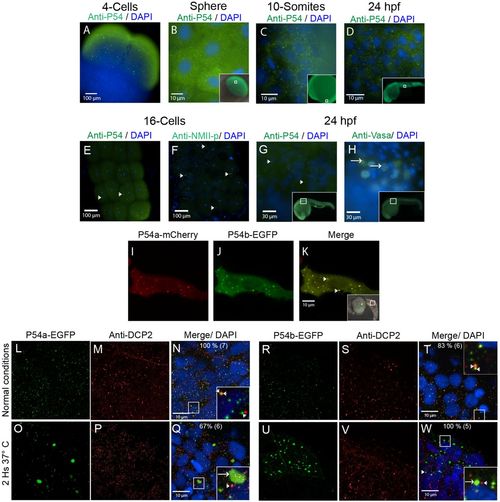
P54 proteins are expressed in cytoplasmic granules during zebrafish development. (A–E,G) Whole-mount immunostaining was performed with the anti-P54 antibody in WT zebrafish embryos at (A) the 4-cell stage, (B) the sphere stage, (C) the 10-somite stage, and (D,E,G) 24 hpf. In these embryos, P54 helicases were located in cytoplasmic granules. Immunostaining (green) and DAPI (blue) were visualized by epifluorescence microscopy. (E–H) P54-positive granules were different from germline granules, observed by immunostaining with anti-NMII-p (16-cell stage) or anti-Vasa (24 hpf), two known markers of germ granules. Arrowheads indicate P54-positive granules, and arrows indicate granules labeled by anti-NMII-p in germplasm or anti-Vasa in germ granules. (I–K) Live embryos at 24 hpf expressing the P54a-mCherry and P54b-EGFP fusion proteins that were also located in cytoplasmic granules. Arrows indicate cytoplasmic granules where P54a and P54b co-localize. (L–W) Expression of P54a-EGFP and P54b-EGFP fusion reporters and immunostaining with anti-Dcp2 (a marker for P-bodies) in 24 hpf embryos maintained at normal temperature (28.5°C) (L–N,R–T) or exposed for 2 h to heat-shock conditions (37°C) (O–Q,U–W). P54a and P54b fusion proteins co-localize with or are in close contact with Dcp2-labeled granules. Under heat-shock conditions, P54a-EGFP and P54b-EGFP were observed in larger granules that can be seen in close contact with smaller Dcp2-positive granules. Small granules, such as P-bodies, are labeled with arrowheads, and larger granules are indicated with arrows. For some images, a general view of the embryos is shown in the inset, where white boxes indicate the regions where the analysis was performed. |
|
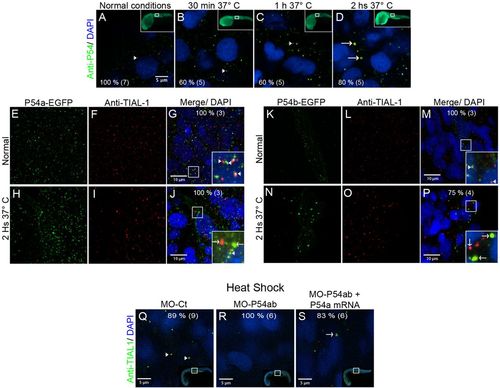
Heat-shock induced the association of P54a and P54b RNA helicases with large cytoplasmic granules. (A,E–G,K–M,Q) Wild-type 24 hpf zebrafish embryos were maintained under normal conditions at 28.5°C or (B–D,H–J,N–P,R,S) under heat-shock conditions at 37°C. (A–D) Anti-P54 immunostaining in embryos subjected to heat-shock for 30 min, 1 h and 2 h. Nuclei are counterstained with DAPI. The top right insets are general views, and the amplified regions are indicated in white boxes. (E–J) Expression of the P54a-EGFP in combination with TIAL-1 immunostaining (as a stress granule marker). In the inset, some P54a-expressing granules co-localize with or are in close contact with TIAL-1-positive granules. (K–P) P54b-EGFP expression in small (at 28.5°C) or large (at 37°C) granules, combined with immunostaining with anti-TIAL-1. The insets show both P54b-expressing granules and TIAL-1-positive granules. Arrowheads point to small granules, such as P-bodies, and arrows indicate larger granules, such as TIAL-1-positive (red) stress granules. (Q–S) Knockdown of P54a and P54b expression by splice-blocking morpholinos prevents the formation of stress granules labeled by anti-TIAL-1. For each panel, the percentage of embryos showing the same pattern is indicated, and the total number of fish embryos tested is shown in parentheses. White arrowheads point to small granules (putative P-bodies) and white arrows to large granules (putative stress granules). |
|
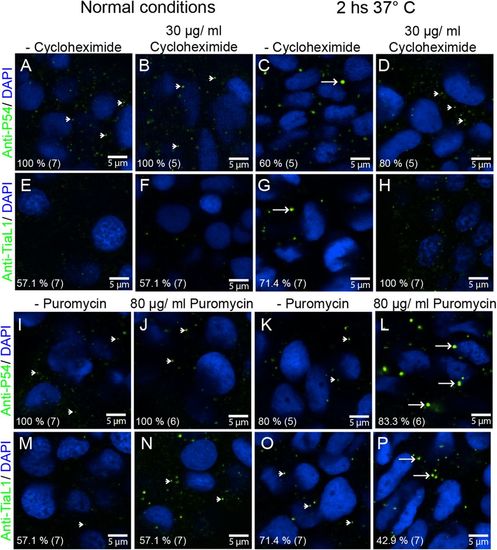
P54 RNA helicase cytoplasmic granules exhibit the properties of stress granules. (A–D,I–L) Anti-P54 immunostaining in 24 hpf WT zebrafish embryos. (E–H,M–P) Anti-TIAL-1 immunostaining as a marker for stress granules in 24 hpf WT embryos. (A–H) Some embryos were treated for 2 h with cycloheximide to block the formation of stress granules. (I–P) Puromycin was added (also for 2 h) to some of the embryos to enhance the assembly of stress granules. (A,B,E,F,I,J,M,N) Fish embryos were maintained at 28.5°C during cycloheximide and puromycin treatments or (C,D,G,H,K,L,O,P) at 37°C. After treatments and immunostaining, fluorescence signals were visualized by confocal microscopy. Arrowheads point to smaller granules (P-body size), and arrows point to larger stress granules. Anti-P54 and anti-TIAL-1 antibodies are both shown in green and DAPI nuclei staining in blue. At the bottom left of each image, the percentage of embryos showing the same immune reaction pattern is indicated, with the total number of embryos treated in parentheses. |
|
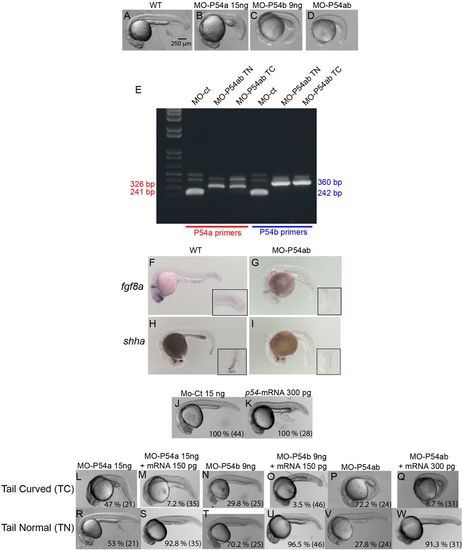
Knockdown of P54a and/or P54b RNA helicases in zebrafish embryos. (A–D) show the defects caused by MO-P54a, MO-P54b or MO-P54ab, which mostly affected posterior trunk structures and produce a bend at the end of the trunk (‘tail curved’). (E) Morpholino efficacy was validated by reverse transcription-PCR (RT-PCR). Using specific primers for p54a or p54b mRNA in samples from embryos micro-injected with MO-ct or MO-P54ab. Primers for amplifying p54a and p54b, under normal conditions, produced bands of 241 and 242 bp, respectively. However, in samples from MO-P54b embryos, bands of 326 or 360 bp were observed due to an intron insertion. (F–I) ISH showing the expression of fgf8a and shha in WT and MO-P54ab 24 hpf embryos. (J,K) There were no obvious defects in embryos micro-injected with 15 ng of MO-ct or 300 pg of p54-mRNA. (L–Q) The ‘tail curved’ phenotype produced by MO-P54a, MO-P54b or MO-P54ab was rescued if the morpholino was co-injected with p54-mRNA. (R–W) With every micro-injection, a proportion of embryos developed normally (‘tail normal’), but this proportion increased drastically upon rescue with p54-mRNA. (J–W) For each image, the percentage of embryos showing that phenotype is indicated, with the total number of embryos analyzed shown in parentheses. Control morpholino (MO-ct), p54a morpholino (MO-P54a), p54b morpholino (MO-P54b), mix of p54a and p54b morpholinos (MO-P54ab), ‘in vitro’ synthesized mRNA from p54a (p54-mRNA). ‘in situ’ hybridization (ISH), tail curved phenotype (TC), tail normal (TN). PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
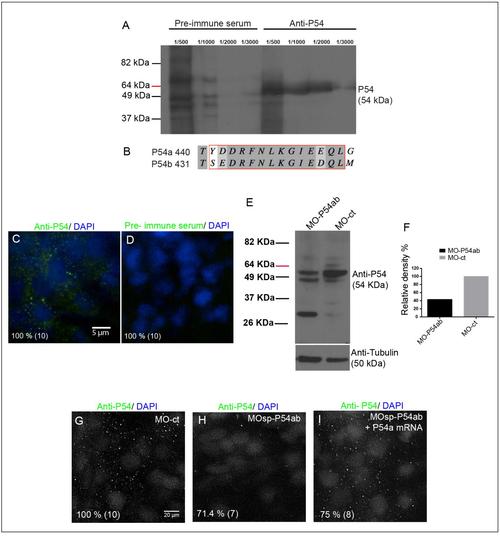
Testing of Anti-P54 serum and P54a and P54b morpholinos. Testing of Anti-P54 serum and P54a and P54b morpholinos. (A) Western blot analysis using protein extracts from 24 hpf WT zebrafish embryos (12 embryos per lane): the rabbit pre-immune serum (dilutions 1/500 – 1/3000) produces no specific signal, whereas the anti-P54 serum (dilutions 1/500 –1/3000) showed a band at approximately 54 kDa, which is the predicted molecular weight of both P54 RNA helicases from zebrafish. (B) Fragment of a protein alignment between P54a and P54b with the sequence used to synthesize the peptide against anti-P54 serum was generated. (C and D) Wholemount immunostaining of WT embryos at 24 hpf using 1/2000 dilution of anti-P54 serum or rabbit pre-immune serum. (E) Protein extracts from 24 hpf embryos micro-injected with MO-P54ab or MO-ct were tested by Western blotting with the anti-P54 serum, and the MO-P54ab embryos show a reduction in the signal. As a loading control, an anti-tubulin antibody was used. (F) Relative density measured in anti-P54 protein bands in both MO-P54ab and MO-Ct total protein samples, normalized using the anti-Tubulin loading controls. (G – I) Immunostaining of MO-ct or MO-P54ab 24 hpf morphants showed the loss of the P54 signal in the MO-P54ab-treated embryos, but this signal could be recovered by rescuing P54a expression by co-injecting a mix of MO-P54ab and mRNA-P54a. Nuclei were counterstained with DAPI. The percentage of embryos showing the same pattern shown in the figure is indicated in each panel, with the total number of embryos tested indicated in parentheses. Control morpholino (MO-ct), mix of p54a and p54b morpholinos (MO-P54ab), “in vitro” synthesized mRNA from p54a (mRNA-P54a). |
|
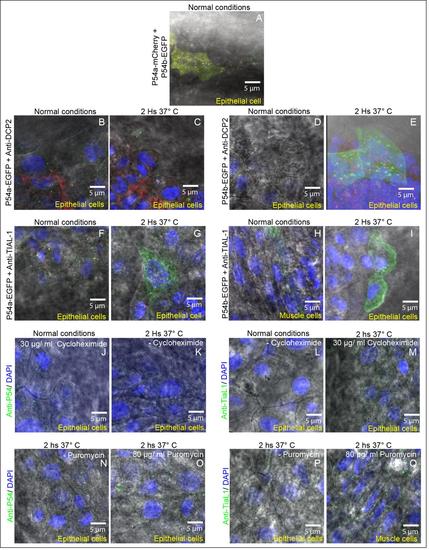
Differential interference contrast (DIC) illumination and cytoplasmic granules in epithelial and muscle cells. In all images we combined DIC illumination and fluorescence microscopy. Nuclei were labeled with DAPI (blue labeling) and cytoplasmic granules with different labels.(A) Cytoplasmic granules of P54a-mCherry and P54b-EGFP fusion reporters in an epithelial cell under normal conditions (same as Fig. 2K). (B and C) Cytoplasmic P54a-EGFP- and anti-Dcp2 labeled granules in an epithelial cell under normal conditions (B same as Fig. 2N) and in heat shock conditions (C same as Fig. 2Q). (D and E) P54b-EGFP- and anti-Dcp2 in cytoplasmic granules in an epithelial cell under normal conditions (D same as Fig. 2T) and in heat shock conditions (E same as Fig. 2W). (F and G) Epithelial cells with cytoplasmic granules containing P54a-EGFP- and labeled with anti-TIAL-1, in normal (F same as Fig. 3G) or heat shock conditions (G same as Fig. 3J). (H) Muscle cell and (I) Epithelial cells, both with cytoplasmic granules containing P54b-EGFP- and anti-TIAL-1, in normal (same as Fig. 3M) or heat shock conditions (same as Fig. 3P), respectively. (J and K) Anti-P54 labeling cytoplasmic granules, in epithelial cells, treated with or without cycloheximide and in normal temperature conditions (same as Fig. 4B) or in heat shock (same as Fig. 4C), respectively. (L and M) Cycloheximide treated and untreated epithelial cells, with anti-P54 labeling cytoplasmic granules, in normal conditions (same as Fig. 4E) or in heat shock (same as Fig. 4H), respectively. (N and O) Cytoplasmic granules labeled with anti-P54 antibody, in epithelial cells, treated with or without puromycin, both in heat shock conditions (same as Fig. 4K and L respectively). (P) Epithelial cell where cytoplasmic granules were labeled with anti-TIAL-1 antibody (same as Fig. 4O) and exposed to a heat shock. (Q) Muscular cell treated with puromycin and in heat shock conditions where cytoplasmic granules were labeled with anti-TIAL-1 antibody. All these images are the same as some Figures 2, 3 and 4 and are presented here with overlapping DIC illumination to observe the type of cell studied. |
|
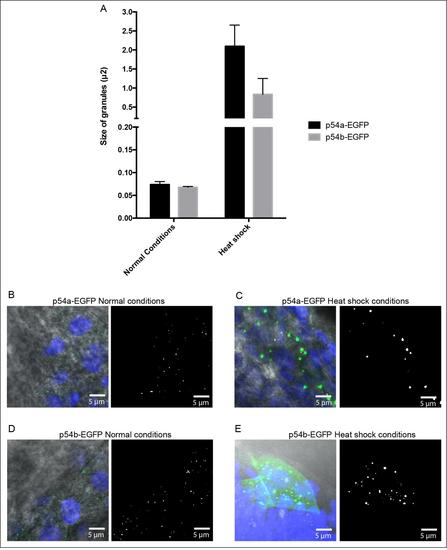
Estimated size for cytoplasmic granules. (A) Graph comparing average area (µm2) for P54a-EGFP and P54b-EGFP cytoplasmic granules. Area values were estimated with ImageJ and later converted to diameter values considering that granules have a circular shape. Under normal temperature P54a-EGFP granules have an average area of 0.07 m2 and a diameter of 0.30 µm2, while P54b-EGFP granules have an average area 0.06 µm2 and a diameter of 0.29 µm2. In heat shock conditions, the average area and diameter for P54a-EGFP cytoplasmic granules was of 2.0 µm2 and 1.63 µm2, respectively. For P54b-EGFP granules, under heat shock the average area and diameter for cytoplasmic granules was of 0.8 µm2 and 1.03 µm2, respectively. (B) Example of P54a-EGFP granules in normal conditions used in our measurements. (C) Example of P54a-EGFP granules in heat shock. (D) P54b-EGFP granules in normal conditions. (E) P54b-EGFP granules in heat shock. |
|
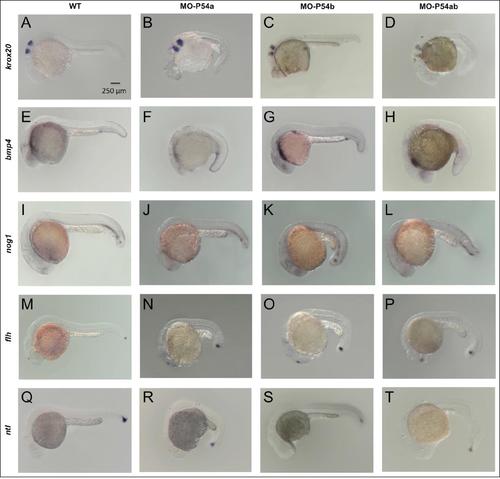
Effects of morpholino knockdown of P54a and P54b RNA helicases in the expression of some markers for zebrafish development. Whole-mount in situ hybridization of WT or morpholino micro-injected 24 hpf zebrafish embryos. (A, E, I, M and Q) show WT embryos. (B, F, J, N and R) Embryos micro-injected with 15 ng of MO-P54a. (C, G, K, O and S) Embryos micro-injected with 9 ng of MO-P54b. (D, H, L, P and T) Embryos micro-injected with a mix of 15 ng of MO-P54a and 9 ng of MO-P54b. Abbreviations: p54a morpholino (MO-P54a), p54b morpholino (MO-P54b), mix of p54a and p54b morpholinos (MO-P54ab), “in situ” hybridization (ISH), early growth response 2a (krox20), bone morphogenetic protein 4 (bmp4), noggin 1 (nog1), floating head or notochord homeobox (flh) and no tail or brachyury homolog (ntl). |