- Title
-
Tumor suppression in basal keratinocytes via dual non-cell-autonomous functions of a Na,K-ATPase beta subunit
- Authors
- Hatzold, J., Beleggia, F., Herzig, H., Altmüller, J., Nürnberg, P., Bloch, W., Wollnik, B., Hammerschmidt, M.
- Source
- Full text @ Elife
|
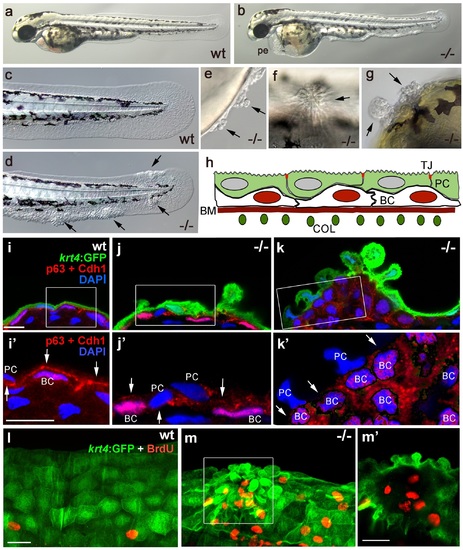
Epidermal aggregates in psoriasis mutants display hyperplasia. (a-g) Live images of wt siblings (a,c) and psoriasis mutants (b,d-g); psoriasis mutants develop pericardial edema (pe) and epidermal aggregates on the medium fin fold (b, d), on the yolk sac (e), on the flank (f) (all at 54 hpf), and on the head (g; 72 hpf). (h) Schematic of embryonic skin. Peridermal cells (PC, green) with apical tight junctions (TJ, red) are located above p63 basal keratinocytes (BC, red nuclei). The basement membrane (BM, red) separates the basal epidermal layer from the dermis containing collagen fibers (COL, green). (i-k) IF of periderm-specific GFP (green), Cdh1 (red), and p63 (red) on transverse sections through 48 hpf Tg(krt4:GFP) wt and psoriasis mutant embryos, counterstained with DAPI (blue). (i′-k′) show magnified views of regions framed in (i-k), without the green channel. In wt, the epidermis is bi-layered, with flat cells (i) and Cdh1 is localized at cell borders between peridermal and basal cells (i′; arrows). In an early-stage aggregate of the mutant, peridermal cells have rounded up (j), and Cdh1 levels are reduced at cell borders (j′,k′; arrows). An advanced aggregate (k) contains multi-layered basal epidermal cells (k′). Scale bar: 10 µm. (l,m) Whole mount IF of incorporated BrdU (red) and periderm-specific GFP (green) in 54 hpf Tg(krt4:GFP) wt sibling (l) and psoriasis mutant (m), showing elevated numbers of BrdU-positive non-peridermal cells in aggregates. (m) Maximum intensity projection of a confocal Z stack through aggregate; (m′) single focal plane. Scale bars: 20 µm. Abbreviations: BC, basal cell; BM, basement membrane; COL, collagen fibers; PC, peridermal cell; pe, pericardial edema; wt, wild-type. EXPRESSION / LABELING:
PHENOTYPE:
|
|
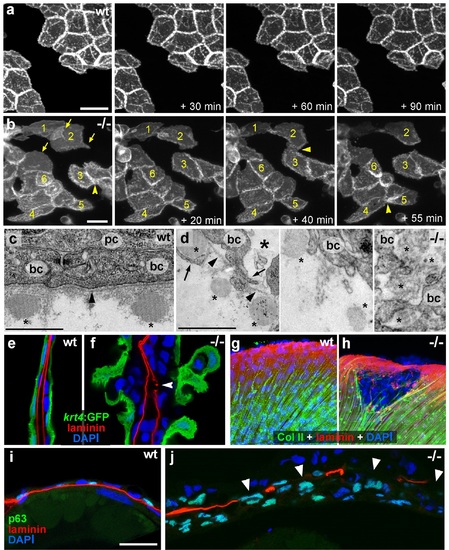
psoriasis keratinocytes display partial EMT and invasive behavior. (a,b) Stills from in vivo time-lapse recordings (Videos 1 and 2) of clones of membrane-bound GFP-labelled (Tg(Ola.Actb:Hsa.hras-egfp)) basal keratinocytes in a mosaic wt (a) or an atp1b1a morphant (b) embryo; ′n min′ indicates elapsed time since the start of the recordings at 48 hpf (Videos 1 and 2). Wild-type cells form a rigid epithelium and maintain their shapes and relative positions (a), whereas atp1b1a morphant cells form cellular processes (arrows), dynamically dis- and re-associate (arrowheads) and eventually crawl on top of each other (cell 1; b, first panel). Cell 1 moves out of the focal plane after 60 min, cell 6 changes its shape from roundish to more hexagonal and vice versa. Scale bars: 20 µm. (c,d) Transverse TEM sections through median fin fold, at 58 hpf. In wt (c), an intact basement membrane (BM; black arrowhead) separates the compact layer of basal cells from the underlying dermis, which contains actinotrichia (small asterisks). The psoriasis mutant (d) displays large intercellular gaps (large asterisk) between basal cells, cellular protrusions (arrows) of basal cells, a discontinued BM (arrowheads to remaining BM), direct contacts between epidermal cells, and disassembling dermal actinotrichia (small asterisks) that lose their regular shape and striated pattern. bc: basal cells; pc: peridermal cell. Scale bars: 1 µm. (e,f) Laminin and peridermal-specific GFP double IF, counterstained with DAPI, at 58 hpf. Transverse sections through the fin fold of Tg(krt4:GFP) transgenics reveal basement membrane fragmentation (arrowhead) below an epidermal aggregate in the mutant (f). (g,h) Laminin and type II collagen double IF, counterstained with DAPI at 58 hpf; view of fin folds of whole mounts, showing basement membrane fragmentation and actinotrichia disassembly in the mutant (h). (i,j) Laminin and p63 double IF, counterstained with DAPI at 58 hpf; transverse section through the yolk sac. Arrowheads in (j) point to holes in the basement membrane of the mutant. Note the presence of p63 keratinocytes below the basement membrane in the dermal space. Scale bar: 20 µm. |
|
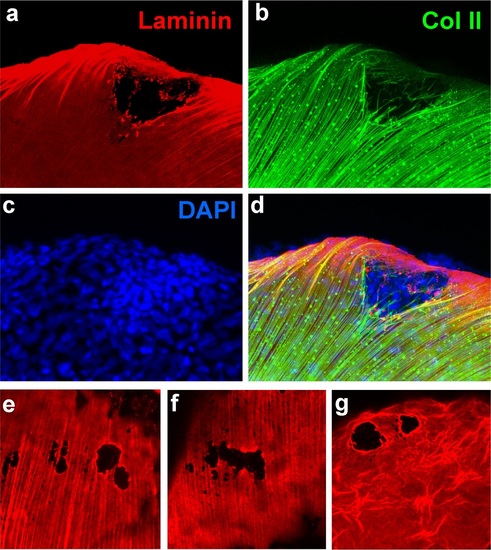
psoriasis mutants display local degradations of the skin basement membrane and of underlying collagenous actinotrichia of the dermis. (a-d) IF of laminin (red; a) and type II collagen (green; b) in a whole mount 58 hpf psoriasis mutant fin fold, counterstained with DAPI (blue; c). The basement membrane is disintegrated (a) and actinotrichia (b) are disassembled below an epidermal aggregate (d; merged channels). (e-g) IFs of laminin (red) show examples of holes in the basement membrane in different 58 hpf psoriasis mutants. |
|
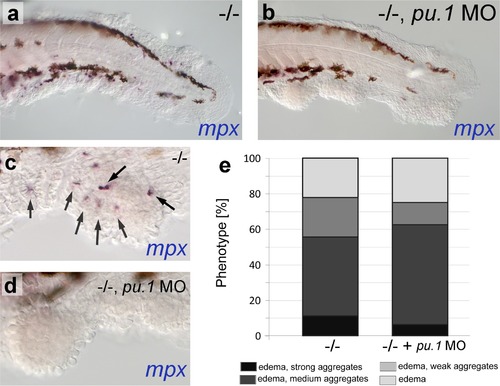
psoriasis mutants display skin inflammation, which does not contribute to the formation of epidermal aggregates. (a-d) WISH of mpx labeling neutrophils in 54 hpf psoriasis-/- and psoriasis-/- ; mpx MO embryos. mpx-positive neutrophils (arrows) have migrated into epidermal aggregates in the psoriasis-/- embryo (a,c). No reduction of epidermal aggregate formation and no mpx-positive neutrophils are observed in psoriasis mutants, in which the myeloid lineage has been ablated by pu.1 MO injection (b, d). (e) Quantification of phenotypes of psoriasis mutants injected with pu.1 MO and un-injected controls. n = 16-18. |
|
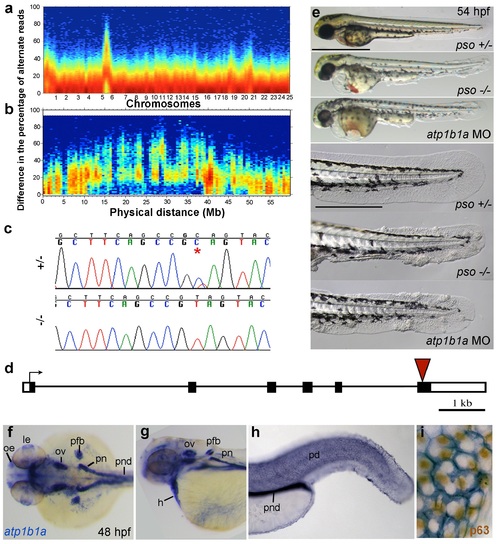
The psoriasis phenotype is caused by a loss-of-function mutation in atp1b1a, which is expressed in multiple epithelia, but not in basal keratinocytes. (a-d) Exome sequencing links the psoriasis mutation to LG6 and identifies a C to T transition in atp1b1a. (a,b) Heat maps showing the density of variant loci over the whole genome (a) and on chromosome 6 (b). The Y-axis shows the absolute value of the difference in the percentage of DNA harboring the variation between the pool of affected offspring and the pool of their parents. Most of the genome shows a difference close to 0%, indicating that the parental DNA segregated randomly in the affected offspring. Only one peak on chromosome 6 shows low density at a difference of 0%, but high density at between 25% and 50% difference, which is expected at the linked locus under the assumption of a recessive mode of inheritance. (c) Chromatographs of Sanger sequencing of sibling and mutant DNA showing the psoriasis mutation (*). (d) Schematic representation of the atp1b1a locus. Red arrowhead indicates the position of the mutation. (e) Live images of 54 hpf wt siblings, psoriasis mutants, and atp1b1a morphants. MO-based knockdown of atp1b1a in wt embryos phenocopies both the pericardial edema and epidermal aggregates. Scale bars: 500 µm; 250 µm (magnifications). (f-i) WISH detects atp1b1a RNA (blue) in heart and multiple epithelia of 48 hpf wt embryos, including the pronephric duct and the periderm but not in the basal keratinocytes (f-h), as seen at higher magnification after counterstaining of nuclei of basal keratinocytes for p63 protein (i). The atp1b1a RNA signal is not detected around p63 nuclei, but is detected in hexagonal peridermal cells with p63- nuclei. Abbreviations: h, heart; le, lense; oe, olfactory epithelium; ov, otic vesicle; pd, periderm; pfb, pectoral fin bud; pn, pronephros; pnd, pronephric duct. |
|
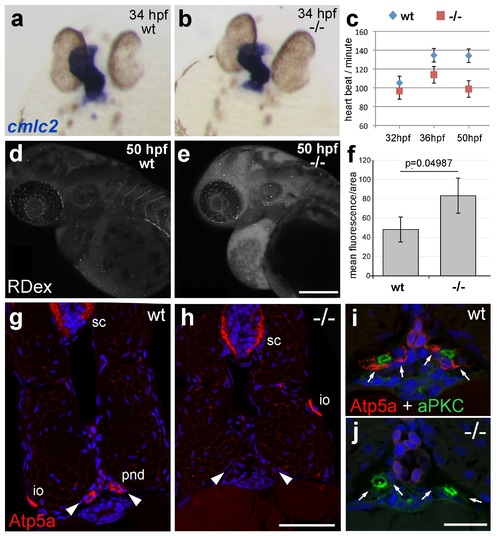
atp1b1a is required for proper heart and pronephric function. (a, b) WISH of cmlc2 in 34 hpf embryos reveals normal heart tube elongation in psoriasis mutants. (c) psoriasis mutants exhibit a reduction of the heart beat. n = 12 (mutants), 24 (siblings). p values: 32 hpf: 3.8E-04, 36 hpf: 6.8E-07, 50 hpf: 1.2E-11. (d- f) psoriasis mutants show compromised clearance / excretion of rhodamine-dextrane injected into the cardinal vein at 34 hpf; confocal images of live embryos of wt sibling (e) and mutant (f) embryos at 50 hpf; RDex, rhodamine-dextrane; scale bar: 200 µm. (f) Quantification of mean fluorescence intensity of a defined area in confocal images, determined with ImageJ software; n = 3 for each condition. Error bars represent standard deviations. (g-j) IF of Atp5a (red) and aPKC (green), counterstained with DAPI (blue), on transverse sections of 54 hpf wt (g,i) and psoriasis mutant (h,j) embryos. Atp5a signal is absent from the pronephric duct (pnd, arrowheads) but not from ionocytes (io) or spinal cord (sc) in psoriasis mutants (g,h). The apical marker aPKC is still present in the pronephric duct cells of psoriasis mutants, outlining the lumen of the ducts, whereas Atp5a is missing from the basolateral site (arrows; i,j). |
|
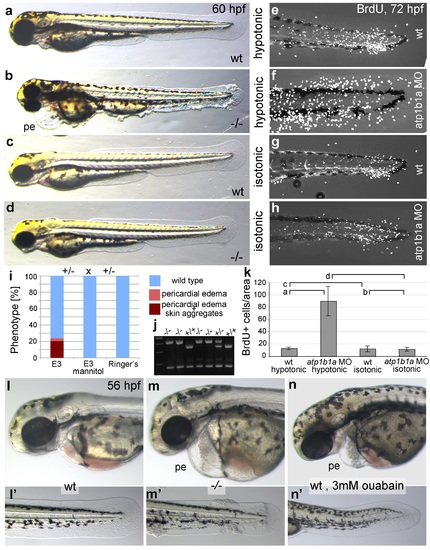
Epidermal hyperplasia is dependent on hypotonic conditions. (a-k) psoriasis mutants raised in isotonic conditions do not develop epidermal hyperplasia. Live images of 60 hpf embryos (a-d) show epidermal aggregates and pericardial edema (pe) in the mutant raised in hypotonic E3 (b) but not in the mutant raised in isotonic E3 (d). IF of BrdU incorporation (white) in 72 hpf wt or atp1b1a morphant tail fins (e-h) reveals excessive cell proliferation in the fin fold of the morphant embryo (f) raised in hypotonic E3 but not in the morphant embryo (h) raised in isotonic E3. (i) Quantification of embryos as shown in (a-d), obtained from incross of two psoriasis /- parents. n = 86-122. (j) Representative gel with PCR products subjected to MwoI restriction digest to genotype embryos raised in isotonic medium. All mutants raised in isotonic medium and shown as representative examples in this and the following figures had been positively genotyped. (k) Quantification of embryos as shown in (e-h), scored is the number of BrdU-positive cells in a given area of the median fin fold. n = 3-7 per condition. Error bars represent standard deviation. p values are as follows: a: 0.0416, b: 0.9139, c: 0.8956, d: 0.0017. (l-n) Live images of 56 hpf wt embryos (l,l′), psoriasis mutant embryos (m,m′), and wt embryos treated with 3mM ouabain (n,n′) starting from 33 hpf. Blockage of Na,K-ATPase pump function by ouabain results in pericardial edema (pe) as in psoriasis mutants (n = 122/125; compare n to m), but not in epidermal aggregates (n = 0/125; compare n′ to m′). PHENOTYPE:
|
|
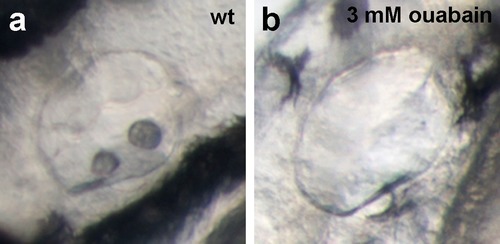
Live images of the otic vesicles of 48 hpf embryos show two otoliths in a untreated wt embryo (a) whereas in an embryo treated with 3 mM ouabain starting from 10 hpf, otoliths have failed to form (b; n = 76/99) or are much smaller (not shown; n = 23/99). |
|
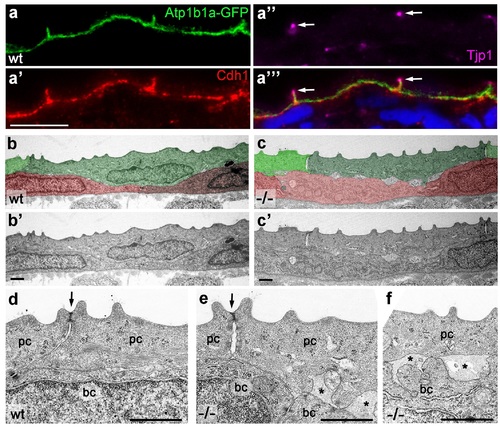
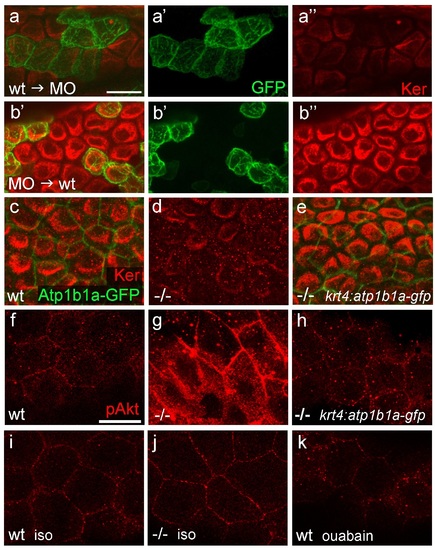
Atp1b1a is required for epidermal cell adhesion. (a) IF of GFP (a, green), Cdh1 (a′, red) and tight junction marker Tjp1 (a′′, magenta) on a transverse section of the epidermis of a 48 hpf wt embryo expressing peridermal-specific krt4:atp1b1a-gfp, counterstained with DAPI (blue; a′′′ with merged channels). Atp1b1a and Cdh1 are co-localized on the basolateral side of peridermal cells, but are excluded from tight junctions and the apical side of these cells. (b-f). Transverse TEM sections through the medium fin fold of wt and psoriasis mutant embryos raised in isotonic conditions, at 58 hpf. In the mutant epidermis (c,c′), aberrant gaps between peridermal cells (false-colored in green) and underlying keratinocytes (false-colored in red) are apparent when compared to the wt epidermis (b,b′). (d-f) Higher magnifications reveal tight junctions (indicated by arrows) of unaltered morphology in the mutant (e) compared to a wt sibling (d), but less organized lateral regions between peridermal cells (e), and large gaps (*) between peridermal and basal cells (e,f) in the mutant. bc, basal cell; pc, peridermal cell. Scale bars: 1 µm. PHENOTYPE:
|
|
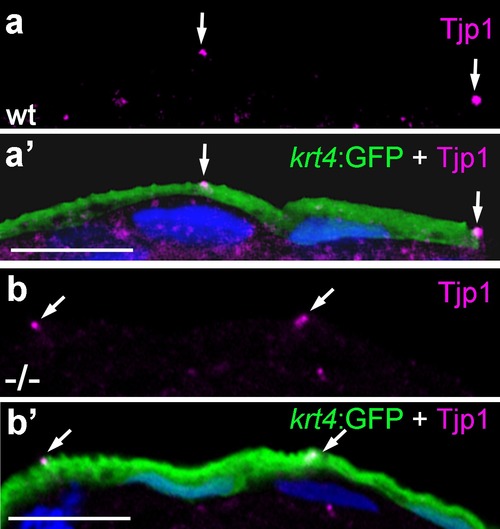
Localization of the tight junction protein Tjp1 is unaltered in psoriasis mutants. IF of Tjp1 (a,b; magenta) and periderm-specific GFP (a′,b′; green; counterstained with DAPI in blue; merged channels); transverse section through epidermis of wt (a,a′) and psoriasis mutant (b,b′) embryos carrying Tg(krt4:GFP) transgene; 48 hpf., Tjp1 (arrowed) shows unaltered apical localization in peridermal cells of mutant. Scale bar: 10 µm. |
|
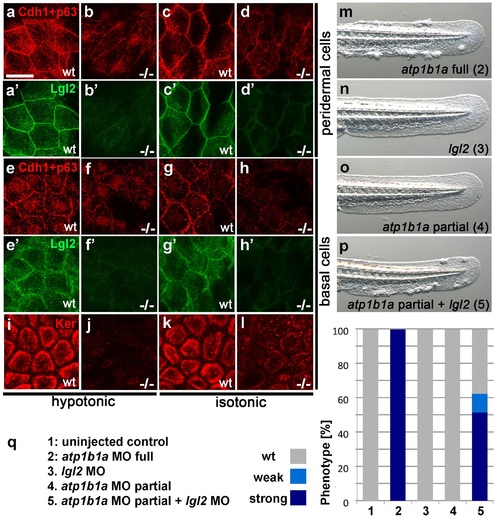
Atp1b1a is required for epidermal integrity and polarity. (a-h). Whole mount IFs of Cdh1 (red) and Lgl2 (green) in 54 hpf embryos raised in hypotonic (a,b,e,f) and isotonic (c,d,g,h) conditions. In mutants, localization of Cdh1 (b, d, f, and h; compare to wt a, c, e, g) and Lgl2 (b′, d′, f′, and h′; compare to wt a′, c′, e′, g′) is compromised in both peridermal cells (a-d) and basal cells (e-h). Images show regions of the trunk epidermis not yet affected by aggregate formation. (i- l). Whole mount IFs of cytokeratin (red) in 84 hpf embryos show a reduction of basally localized cytokeratin in mutants raised in hypotonic (j) and isotonic (l) conditions. Images show regions of the trunk epidermis above the yolk sac extension. (m-p). Live images of the tail fins of 54 hpf embryos either with full MO knockdown of atp1b1a (m) or with partial MO knockdown of lgl2 (n), of atp1b1a (o), or of both (p). (q) Quantification of the phenotypes of 54 hpf embryos in synergistic enhancement studies; n = 31-88. Similar results were obtained in two additional independent experiments. EXPRESSION / LABELING:
PHENOTYPE:
|
|
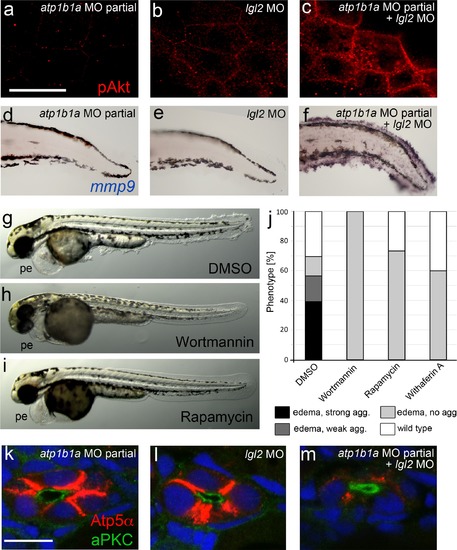
atp1b1a and lgl2 interact genetically to enhance edema formation and AKT phosphorylation and mmp9 expression in basal keratinocytes of embryos raised in hypotonic medium. (a-c) pAKT IF, at 54 hpf, revealing low pAKT levels in embryos injected with low amounts of atp1b1a MO (a) or with lgl2 MO (b), but strongly increased levels in a double-injected embryo (c). For comparison with pAKT levels in wild-type and psoriasis mutants (full loss of Atp1b1a activity), see Figure 8f,g. (d-f). mmp9 WISH, at 54 hpf, revealing low mmp9 expression in embryos injected with low amounts of atp1b1a MO (d) or with lgl2 MO (e), but strongly increased levels in double-injected embryo (f). For comparison with mmp9 expression levels in wild-type and psoriasis mutant, see Figure 9j,j′′. (g-j). Embryos co-injected with sub-phenotypic amounts of atp1b1a MO and lgl2 MO display epidermal aggregates in conjunction with pericardial edema. Treatment with the PI3K inhibitor Wortmannin, the mTORC1 inhibitor Rapamycin or the NFºB inhibitor Withaferin A, starting at 34 hpf, leads to a complete loss of epidermal aggregates, whereas edema persist. (g-i) Representative live images of DMSO-treated controls (g), and of Wortmannin-treated (h) and Rapamycin-treated (i) embryos, at 54 hpf; pe, pericardial edema. (j) Quantification of the phenotypes of control, Wortmannin-, Rapamycin- or Withaferin A-treated embryos as shown in (g- i), at 54 hpf. For classification of phenotypic strengths, see Figure 9a-d. (k-m). IF of Atp5a (red) and aPKC (green), counterstained with DAPI (blue), on transverse sections at 54 hpf, revealing high amounts of Na,K-ATPase α-subunits in the basolateral membrane domains of pronephric duct epithelial cells in embryos injected with low amounts of atp1b1a MO (k) or with lgl2 MO (l), but strongly decreased levels in double-injected embryo (m). Scale bar:10 µm. |
|
atp1b1a is required in peridermal cells to establish epithelial organization of basal keratinocytes, and to suppress hypotonicity-induced upregulation of pAKT levels in basal keratinocytes. (a-b) IF of cytokeratins (Ker; red) and GFP (green) in 84 hpf chimeric embryos raised in isotonic conditions. Wild-type (wt) basal keratinocytes expressing Tg(Ola.Actb:Hsa.hras-egfp)-encoded membrane-tagged GFP transplanted into atp1b1a morphant hosts display reduced cytokeratin localization similar to that of host cells (a), whereas the cytokeratin distribution of morphant donor cells (green) in wt hosts is indistinguishable from that in neighboring wt cells (b). Images show regions of the trunk epidermis above the yolk sac extension. (c-e). Maximum intensity projections of confocal images of IF of cytokeratin (red) and periderm-specific Atp1b1a-GFP (green) in 84 hpf embryos obtained from an in-cross of psoriasis +/- ; Tg(krt4:atp1b1a-gfp) parents, raised in isotonic conditions. Embryos were genotyped after imaging. Cytokeratin localization in basal keratinocytes is distorted in non-transgenic psoriasis-/- embryos (d; compare to wt in c), but restored in psoriasis-/- ; Tg(krt4:atp1b1a-gfp) embryos (e). Images show regions of the trunk epidermis above the yolk sac extension. (f-k) pAKT IF (red) in 54 hpf embryos. pAkt is upregulated in psoriasis mutants raised in hypotonic (g) but not in isotonic (j) medium, compared to wt siblings (f, i). pAkt levels are ameliorated in psoriasis-/- ; Tg(krt4:atp1b1a-gfp) embryos kept in hypotonic medium (h). pAkt is not upregulated in wt embryos incubated in hypotonic medium after addition of 3 mM ouabain, starting from 33 hpf (k). Images show regions of the trunk epidermis not yet affected by aggregate formation. Abbreviation: iso, isotonic medium (E3 250 mM mannitol). EXPRESSION / LABELING:
PHENOTYPE:
|
|
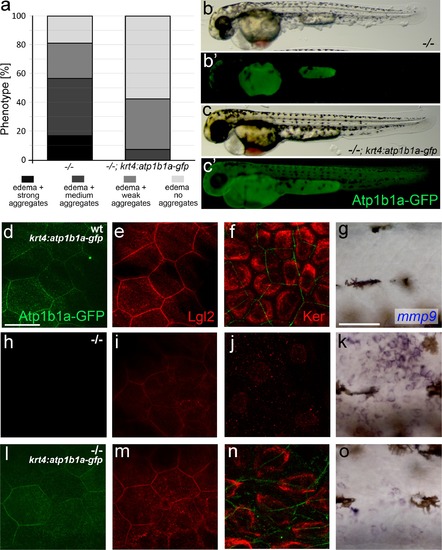
Tg(krt4:atp1b1a-gfp)-driven atp1b1a expression in the periderm of psoriasis mutants kept in hypotonic medium leads to a partial rescue of the polarity defects and malignant transformation in basal keratinocytes. (a) Quantification of phenotypes of non-transgenic psoriasis mutants and psoriasis mutants carrying the krt4:atp1b1a-gfp transgene, raised in hypotonic E3, at 54 hpf. For classification of phenotypic strengths, see Figure 9a-d. (b-c) Representative live images of a psoriasis mutant lacking the transgene (no peridermal GFP; b′), which has pericardial edema and epidermal aggregates (b), and a psoriasis mutant expressing the transgene (peridermal GFP; c′), which has pericardial edema but no epidermal aggregates (c). (d-o) IF of GFP (transgene-encoded Atp1b1a-GFP) in periderm (green; d,h,l; 54 hpf), Lgl2 in periderm (red; e,i,m; 54 hpf) and cytokeratin in basal keratinocytes (red; f,j,n; 84 hpf), and WISH of mmp9 transcripts in basal keratinocytes (g,k,o; 54 hpf) of transgenic wt siblings (d-g), non-transgenic psoriasis mutants (h-k) and psoriasis mutants carrying the krt4:atp1b1a-gfp transgene (l-o). Periderm-specific expression of atp1b1a in mutant embryos leads to a partial restoration of the polarity markers Lgl2 and cytokeratin, and to a reduction of the malignancy marker mmp9. However, obtained levels are still higher than in the wt sibling control. For the reduction of the malignancy marker pAKT, see Figure 8f-h. Scale bars: 20 µm (d-f, h-j. l-n) and 50 µm (g, k, o). |
|
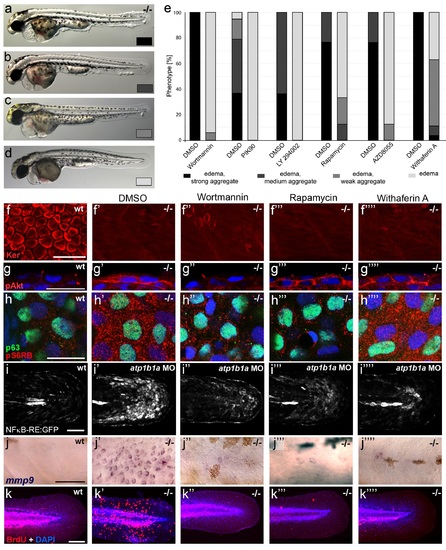
Hyperplasia and transcriptional upregulation of mmp9 in basal keratinocytes of psoriasis mutants is mediated via an aberrant activation of a linear PI3K-Akt-mTorC1-NFκB pathway. (a-e) Blockade of PI3K, mTorC1, and NFκB signaling rescues epidermal aggregate but not pericardial edema formation in psoriasis mutants. (a-d) Representative live images of phenotypic strength classes of psoriasis -/- embryos at 54 hpf, all with pericardial edema of comparable strengths, but strong (a), intermediate (b), weak (c), or no (d) epidermal aggregates. (e) Quantification of the phenotypes of psoriasis mutants incubated in E3 medium containing 1 µM Wortmannin, 5 µM PIK90, 25 µM LY94002, 1.1 µM Rapamycin, 30 µM AZD8055, or 30 µM Withaferin A compared to the corresponding DMSO controls (n = 16-30). Drugs were added at 34 hpf and embryos scored at 54 hpf. Similar results were obtained in at least two additional independent experiments. For representative live images, see Figure 9- figure supplement 1. f-k. A linear PI3K-Akt-mTORC1-NFκB pathway mediates hyperplasia and upregulation of mmp9 expression in basal keratinocytes. All embryos had been kept in (hypotonic) E3 medium, supplemented with the indicated drugs starting at 34 hpf. (f--f′′′′) IF of cytokeratins (red) at 84 hpf. Distorted keratin localization in the psoriasis mutant (f′, compare to wt (f)) is not restored by Wortmannin (f′′), Rapamycin (f′′′), or Withaferin A (f′′′′). Scale bar: 50 µm. (g-g′′′′) IF of pAkt (red), counterstained with DAPI (blue); transverse sections of 54 hpf psoriasis mutants raised in E3 medium. Elevated pAkt levels in the mutant (g′, compared to the wt (g)) are lowered by Wortmannin (g′′), but not by Rapamycin (g′′′) or Withaferin A (g′′′′). Scale bar: 20 µm. (h-h′′′′) IF of pS6RP and p63 of whole mounts, at 54 hpf. Elevated pS6RP levels in mutant (h′, compared to wt (h)) are alleviated by Wortmannin (h′′), PIK90 (not shown) and Rapamycin (h′′′), but not by Withaferin A (h′′′′). Scale bar: 20 µm. (i- i′′′′) Confocal images of GFP fluorescence in the tail fin of a live 48 hpf wt embryo (i) and an atp1b1a morphant (i′), both carrying the Tg(NFκB-RE:eGFP) transgene. The atp1b1a morphant shows strong upregulation of NFκB activity in keratinocytes, which is restored by treatment with Wortmannin (i′′), Rapamycin (i′′′), and Withaferin A (i′′′′). Scale bar: 100 µm. For quantification, see Figure 9-figure supplement 2. (j-j′′′′) mmp9 WISH at 54 hpf. Elevated mmp9 expression in the mutant epidermis (j′, compare to wt (j)) is downregulated by Wortmannin (j′′), Rapamycin (j′′′), and Withaferin A (j′′′′). Scale bar: 50 µm. (k-k′′′′) IF of incorporated BrdU (red), counterstained with DAPI (blue) at 56 hpf. Elevated cell proliferation in mutant epidermis (k′, compare to wt (k)) is downregulated by Wortmannin (k′′), Rapamycin (k′′′), and Withaferin A (k′′′′). Scale bar: 100 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
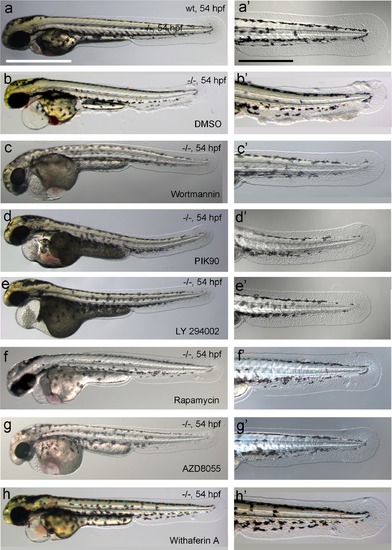
Chemical inhibiton of PI3K, mTORC1 or NFkB rescues the epidermal malignancies, but not the pericardial edema of psoriasis mutants. (a-h) Representative live images of psoriasis -/- embryos at 54 hpf, treated with the PI3K inhibitors Wortmannin (1 µM; c,c′), PIK90 (5 µM; d,d′) and LY 294002 (25 µM; e,e′), the mTORC1 inhibitors Rapamycin (1.1 µM; f,f′) and AZD8055 (30 µM; g,g′), and the NFκB inhibitor Withaferin A (30 µM; h, h′), as quantified in Figure 9e. Treatment with the different inhibitors of components of the PI3K-Akt-mTorC1-NFκB pathway do not rescue the pericardial edema (c- h, compare to wt (a) and non-treated mutant (b)), but do rescue the epidermal malignancies of mutant embryos (c′-h′, compare to wt (a′) and non-treated mutant (b′)). Scale bars: 500 µm (a), 250 µm (a′). |
|
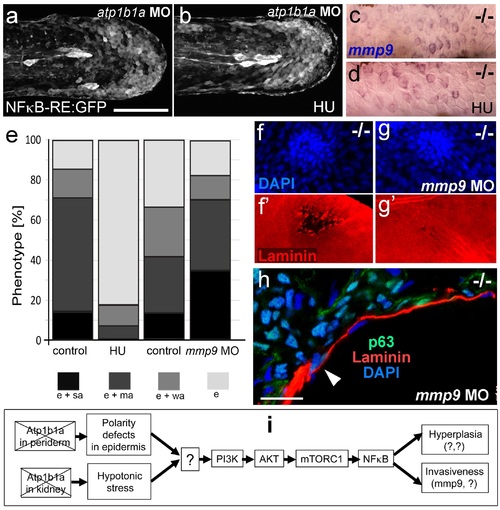
Blockage of cell proliferation results in the normalization of epidermal hyperplasia, whereas blockage of Mmp9 activity reduces epidermal invasiveness in psoriasis mutants. (a-b).Confocal images of GFP in the tail fin of live 48 hpf atp1b1a morphant Tg(NFκB-RE:eGFP) transgenics, showing that elevated NFκB activity in atp1b1a morphants (a) is not reduced by hydroxyurea treatment (b). For quantification, see Figure 10-figure supplement 1. (c-d) mmp9 WISH of 54 hpf psoriasis mutants raised in hypotonic E3. Elevated mmp9 expression in mutant epidermis (c) is not reduced by hydroxyurea (HU) treatment (d). (e) Quantification of the phenotypes of psoriasis mutants, either treated with 50 mM hydroxyurea or injected with mmp9 MO, compared to their respective siblings. e, pericardial edema; wa, weak epidermal aggregates; ma, medium epidermal aggregates; sa, strong epidermal aggregates . n = 17-47. Similar results for each condition were obtained in two additional independent experiments. (f-g) mmp9 knockdown alleviates basement membrane fragmentation. Laminin IF, counterstained with DAPI, in psoriasis mutants at 58 hpf, epidermal aggregates of comparable sizes. In the un-injected psoriaris mutant (f), the aggregate is associated with BM fragmentation, while the underlying BM is largely intact in the psoriasis mutant injected with mmp9 MO (g). For more images and numbers, see Figure 10-figure supplement 2. (h) mmp9 knockdown alleviates epidermal invasiveness. Laminin and p63 IF of transverse sections, counterstained with DAPI, through the yolk sac of a psoriasis mutant (58 hpf) injected with mmp9 MO. The basement membrane is largely intact (arrowhead to small remaining region with thinner basement membrane), and p63 keratinocytes are confined to the epidermal compartment above the basement membrane. For un-injected mutant and wt controls, see Figure 2i,j. (i) Diagram of the identified pathway in which the two required non-cell-autonomous effects caused by loss of Atp1b1a in periderm and osmoregulatory organs converge in basal cells. The pathway subsequently diverges downstream of NFκB to mediate overgrowth versus invasiveness of transformed keratinocytes. Question marks indicate components that have not yet been identified. For details, see text. |
|
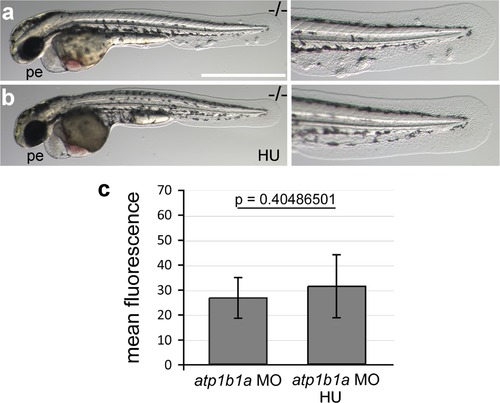
Morphology rescue of psoriasis mutant upon treatment with hydroxyurea and quantification of the non-alleviating effect of the treatment on NFκB activity in embryos as shown in Figure 10a,b. (a-b) Live images of a 54 hpf psoriasis mutant treated with 50 mM hydroxyurea (HU) in E3 from 34 hpf onwards (b) and control mutant raised in E3 (a). Hydroxyurea does not rescue the pericardial edema (a,b), but blocks epidermal aggregate formation (a′,b′). (c) Mean fluorescence intensities of GFP in the posterior part of the tail fin were measured in maximum intensity projections of confocal images using the ImageJ software. n = 7-8. Error bars represent standard deviation. |
|
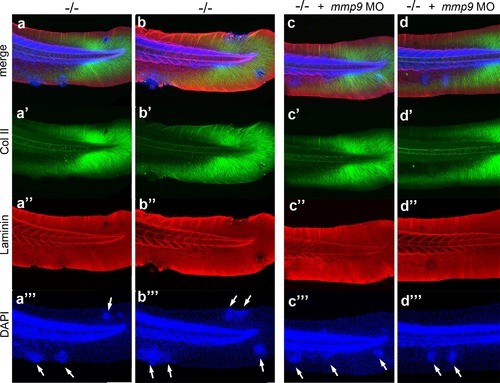
mmp9 knockdown alleviates epidermal invasiveness. Examples of whole mount immunofluorescence of laminin (red) and type II collagen (green) of the tail fin of 58 hpf psoriasis-/- embryos (a,b) and psoriasis-/- ; mmp9 MO embryos (c,d)-, counterstained with DAPI (blue). Actinotrichia disassembly (a′-d′) and basement membrane disruption (a′′-b′′) observed below epidermal aggregates (arrows in a′′′-d′′′) are strongly reduced upon knockdown of mmp9. In total (5 embryos examined per condition), 5/22 medium-sized fin fold aggregates were associated with BM fragmentation in mmp9 MO-injected mutants, compared to 19/19 in un-injected mutant controls. |