- Title
-
Functional Investigation of a Non-coding Variant Associated with Adolescent Idiopathic Scoliosis in Zebrafish: Elevated Expression of the Ladybird Homeobox Gene Causes Body Axis Deformation
- Authors
- Guo, L., Yamashita, H., Kou, I., Takimoto, A., Meguro-Horike, M., Horike, S.I., Sakuma, T., Miura, S., Adachi, T., Yamamoto, T., Ikegawa, S., Hiraki, Y., Shukunami, C.
- Source
- Full text @ PLoS Genet.
|
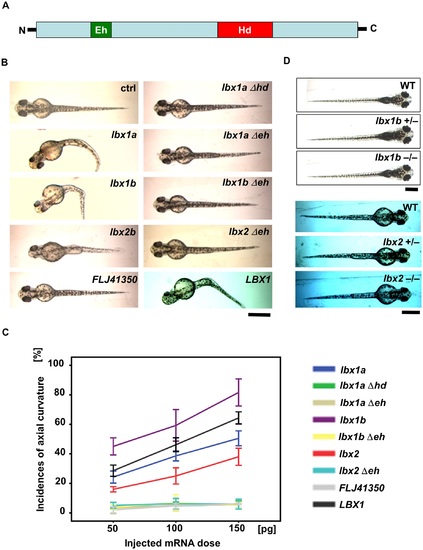
Body curvature induced by injection of lbx mRNAs. (A) Protein structure of Lbx family members with an engrailed homology domain (Eh) and homeobox domain (Hd). (B) Dorsal views of live embryos (48 hpf) injected with zebrafish lbx1a, lbx1b, or lbx2b mRNA, as well as human LBX1 or FLJ41350 mRNA, in comparison with those injected with lbx1aΔhd mRNA which lacks the homeodomain, and lbx1aΔeh, lbx1bΔeh, and lbx2Δeh mRNA which lacks the engrailed domain. Buffer was injected as a control (ctrl). Severe curvature was observed in embryos injected with lbx1a, lbx1b, and LBX1 mRNA, while mild body curvature was observed in those injected with lbx2 mRNA, but no curvature was observed in those injected with lbx genes lacking the functional domains. Bending of the body axis was not observed in embryos injected with FLJ41350. (C) Quantitative analysis of the phenotype of embryos injected with different doses of mRNA. Body curvature occurred in lbx1b, LBX1, lbx1a, and lbx2 embryos in a dose-dependent manner. lbx1b embryos presented with the highest incidence of curvature (45% for 50 pg, 59% for 100 pg, and 82% for 150 pg), and then LBX1 (29% for 50 pg, 46% for 100 pg, and 64% for 150 pg), lbx1a (24% for 50 pg, 39% for 100 pg, and 51% for 150 pg), and lbx2 (16% for 50 pg, 25% for 100 pg, and 38% for 150 pg). The incidence of curvature was less than 10% in lbx1aΔhd, lbx1aΔeh, lbx1bΔeh, lbx2Δeh, and FLJ41350 embryos regardless of dose. The data represent the mean ± standard error of three independent injections (n = 27–37). (D) Dorsal views of live larva are shown. lbx1b+/- and lbx1b-/- mutants as well as lbx2+/- and lbx2-/- mutants are comparable to WT with a straight body axis. The scale bar represents 500 µm. PHENOTYPE:
|
|
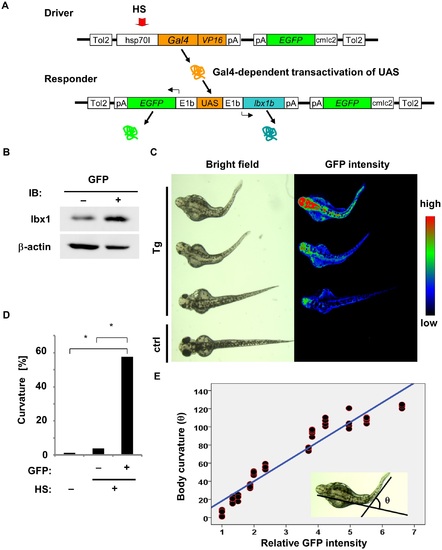
Body curvature induced by lbx1b overexpression under the control of the heat shock-inducible hsp70I promoter. (A) The constructs in the Gal4/UAS—based bidirectional expression system. The F0 driver transgenic carriers were crossed with the F0 responder transgenic carriers to produce Tg(hsp:Gal-VP;EGFP:UAS:lbx1b) F1. Heat shock (HS) treatment activated the hsp70I promoter in the transgene driver (Driver), which expresses Gal4-VP16. Gal4-VP16 protein bound to UAS on the transgene responder (Responder) and activates the expression of lbx1b and EGFP via E1b minimal promoters. (B) Expression of lbx1 protein in 48 hpf transgenic zebrafish with (GFP+) or without HS (GFP-). (C) Body curvature of transgenic 48 hpf zebrafish that received HS at 4 hpf. The severity of body curvature was correlated with GFP intensity. (D) Incidence of body curvature at 48 hpf in embryos with (HS+EGFP+, n = 85) and without (HS+EGFP-, n = 129) EGFP expression and without HS (n = 79). The incidence of curvature was significantly increased in the HS+EGFP+ embryos (*p < 0.01). (E) lbx1b expression level and body curvature. A linear regression line was obtained between the relative fluorescence intensity of GFP and the severity of body curvature quantified by the angle θ: [θ/degree] = 21.70 × [relative fluorescence intensity]– 3.37, with the slope coefficient different from 0 (p < 0.01). This model accounted for 92.2% of the θ variance. PHENOTYPE:
|
|
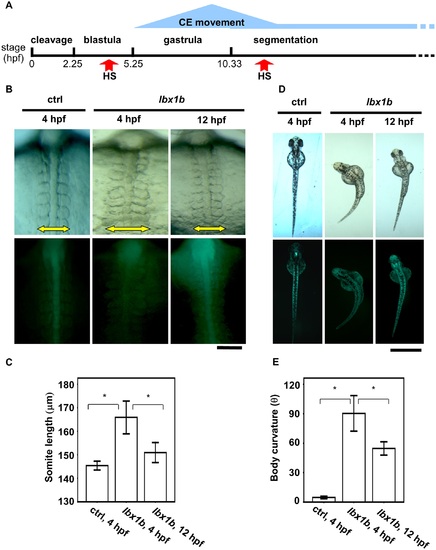
Time-dependent induction of somite mediolateral elongation and body curvature by lbx1b overexpression. (A) A schematic diagram of zebrafish early developmental stages. Convergent extension (CE) is mainly involved in gastrulation. (B) Comparison of somite mediolateral length (16 hpf) between Tg(hsp:Gal-VP; UAS:EGFP) with heat shock (HS) treatment at 4 hpf (ctrl 4 hpf), Tg(hsp:Gal-VP; EGFP:UAS:lbx1b) with HS at 4 hpf (lbx1b 4 hpf), and Tg(hsp:Gal4-VP; EGFP:UAS:lbx1b) with HS at 12 hpf (lbx1b 12 hpf). A significant elongation of somite mediolateral length (yellow arrow in the upper panels) was observed in the lbx1b embryos at 4 hpf. The scale bar represents 100 µm. (C) Quantitative analysis of somite mediolateral length in embryos at 16 hpf. A significant difference was observed between ctrl at 4 hpf (n = 16) and lbx1b at 4 hpf (n = 13), and lbx1b at 4 hpf (n = 13) and lbx1b at 12 hpf (n = 14), *p < 0.01. (D) Comparison of body curvature (48 hpf) between Tg(hsp:Gal-VP; UAS:EGFP) with HS treatment at 4 hpf (ctrl 4 hpf), Tg(hsp:Gal-VP:EGFP:UAS:lbx1b) with HS at 4 hpf (lbx1b 4 hpf), and Tg(hsp:Gal-VP:EGFP:UAS:lbx1b) with HS at 12 hpf (lbx1b 12 hpf). More severe curvature of the body axis was induced in lbx1b embryos at 4 hpf than in lbx1b embryos at 12 hpf. The scale bar represents 1 mm (E) Quantitative analysis of body curvature at 48 hpf. A significant difference was observed between ctrl 4 hpf (n = 13) and lbx1b 4 hpf (n = 10), and between lbx1b 4 hpf (n = 10) and lbx1b 12 hpf (n = 11), *p < 0.05. The severity of body curvature was quantified by the angle described in the legend of Fig 3. Both driver transgenic and responder transgenic fish were F2 lines. PHENOTYPE:
|
|
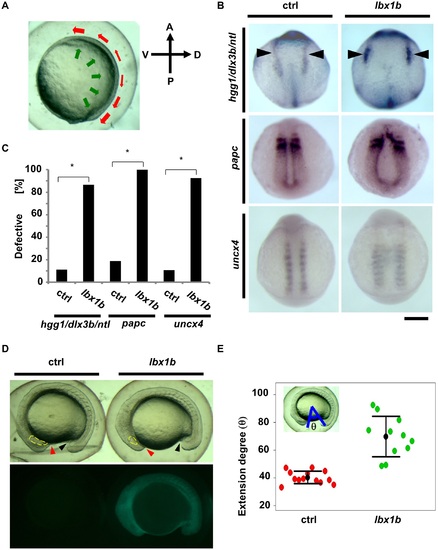
Defective convergent extension in lbx1b overexpressing zebrafish embryos. (A) A diagram of convergent extension during zebrafish gastrulation. A, P, D, and V indicate anterior, posterior, dorsal, and ventral sides, respectively. The green and red arrows indicate the directions of convergence and extension movements, respectively. (B) In situ hybridization for germ layer markers at the early stages of development. Dorsal views of embryos injected with buffer as control (ctrl) or lbx1b mRNA (lbx1b). Upper panels show marker expression of hgg1 (rostral mesoderm), ntl (notochord), and dlx3b (border between neural and non-neural ectoderm, black arrowheads) at the tail bud stage. Middle panels show the expression of papc (paraxial mesoderm) at 11 hpf. Lower panels show the expression of uncx4 (somite) at 13 hpf. The scale bar represents 200 µm. (C) Incidence of defective expression patterns. Significant differences (*p < 0.01) were observed for the germ layer markers. The numbers of control and lbx1b zebrafish embryos were 36 and 30 for hgg1/ntl/dlx3b, 32 and 28 for papc, and 28 and 27 for uncx4, respectively. (D) Lateral views of 16 hpf normal sibling (ctrl) and Tg(hsp:Gal4-VP16:EGFP:UAS:lbx1b) (lbx1b) embryos upon heat shock at 4 hpf. The red and black arrowheads indicate the border between the yolk and the rostral part and the yolk and the caudal part, respectively. The yellow lines indicate the developing eyes. (E) Quantitative analysis of extension movement. Progression of the movement was quantified by the angle θ at 16 hpf for sibling control (ctrl; n = 13) and transgenic (lbx1b; n = 11) embryos. Convergent extension movement was significantly delayed in lbx1b embryos (p < 0.01). PHENOTYPE:
|
|
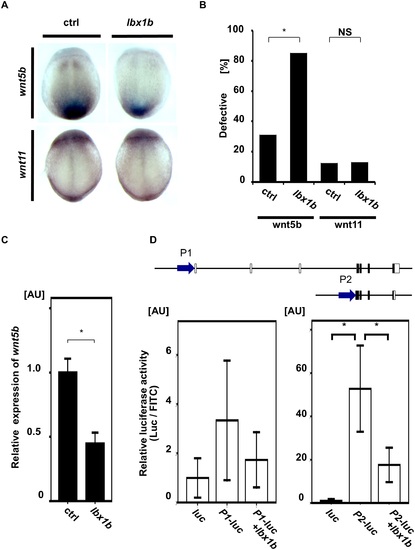
Downregulation of wnt5b during gastrulation by lbx1b overexpression. (A) In situ hybridization for non-canonical Wnt/PCP ligands (wnt5b and wnt11) in control and lbx1b-overexpressing embryos at 90% epiboly. Dorsal views (anterior to the top) for wnt5b and wnt11 of embryos injected with buffer (ctrl) or lbx1b mRNA (lbx1b). (B) Incidence of the defective expression patterns observed in the embryos shown in panel A. Significant differences (*p < 0.01) were observed for wnt5b. The numbers of control and lbx1b zebrafish embryos were 22 and 21 for wnt5b and 23 and 22 for wnt11, respectively. NS: not significant. (C) Decreased wnt5b expression in 90% epiboly embryos injected with lbx1b mRNA by quantitative RT-PCR assays. *p < 0.01. (D) In vivo luciferase assay in 90% epiboly embryos injected with control vector (luc) or the putative promoter regions (P1 and P2) of zebrafish wnt5b. P2 showed higher transcriptional activity than P1. Co-injection of lbx1b mRNA in P2 significantly repressed the transcriptional activity. *p < 0.01. AU, arbitrary unit. |
|
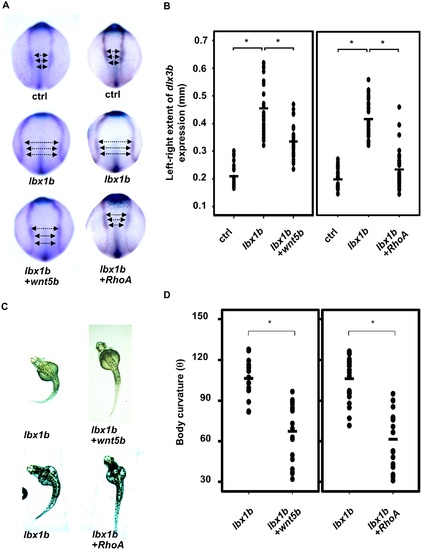
Rescue of defective convergent extension in lbx1b-overexpressing embryos by wnt5b and RhoA mRNA injection. (A) Dorsal view of dlx3b/hgg1/ntl expression in tail bud embryos injected with buffer (ctrl), lbx1b mRNA (lbx1b), lbx1b plus zebrafish wnt5b mRNA (lbx1b+wnt5b), or lbx1b plus human RhoA mRNA (lbx1b+RhoA). (B) Quantitative analysis of convergent extension movement in the tail bud embryos in (A). (ctrl, n = 26 and 29; lbx1b, n = 27 and 33; lbx1b+wnt5b, n = 27; lbx1b+RhoA, n = 32). The extent of defective convergent extension was evaluated by measuring the distance between the inner edges of bilateral dlx3b expression at 3 regions as indicated by the arrows in panel A. Wnt5b or RhoA mRNA injection significantly rescued defective convergent extension (*p < 0.01). (C) Dorsal view of 48 hpf Tg(hsp:Gal-VP;EGFP:UAS:lbx1b) embryos upon heat shock at 4 hpf with buffer (lbx1b), wnt5b mRNA (lbx1b+wnt5b), or RhoA mRNA (lbx1b+RhoA) injection. (D) Quantitative analysis of body curvature in Tg(hsp:Gal4-VP;EGFP:UAS:lbx1b) embryos upon heat shock at 4 hpf with buffer injection (lbx1b, n = 16 and 21; lbx1b+wnt5b, n = 18; lbx1b+RhoA, n = 18).The severity of body curvature was significantly alleviated in Wnt5b or RhoA mRNA-injected embryos with heat shock at 4 hpf, *p < 0.01. Severity of body curvature was quantified by the angle as described in Fig 3. F2 lines of driver transgenic and responder transgenic fish were used. Injections were performed at doses of 50 pg/embryo for lbx1b mRNA, 40 pg/embryo for wnt5b mRNA, and 15 pg/embryo for RhoA mRNA. |
|
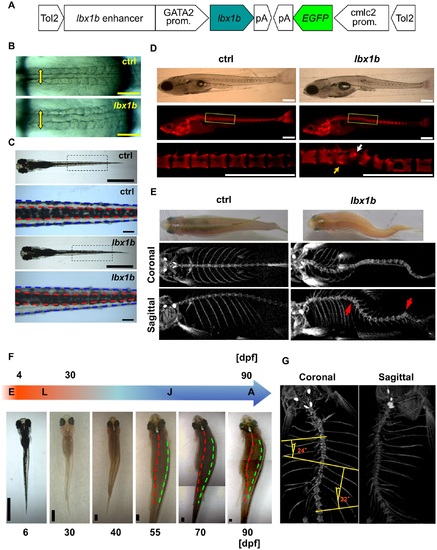
Scoliosis in transgenic founder zebrafish expressing lbx1b using the GATA2 minimal promoter and the lbx1b enhancer. (A) The transgene construct is shown. The GATA2 minimal promoter and lbx1b enhancer cooperatively drive lbx1b expression. (B) Dorsal views of live GATA2-1b:MCS (ctrl) or GATA2-1b:lbx1b (lbx1b) injected embryos with 10–13 somites. Somite arrangement is bilateral and symmetric in control embryos, but asymmetrical in lbx1b embryos. The yellow arrows indicate the mediolateral length of somites. (C) Dorsal views of 6 dpf larvae. The red and blue dotted lines indicate the boundaries of the dorsal melanophore stripes and the trunk, respectively. A displaced dorsal melanophore stripe was observed in lbx1b larvae. (D) Lateral views of alizarin red-stained larvae at 21 dpf. The white and yellow arrows indicate the hemivertebrae and block vertebra that developed from the deformed notochord in lbx1b larva, respectively. (E) Dorsal views and micro-computed tomography (µCT) analysis of adult zebrafish. Scoliosis was observed in adult fish grown from embryos with mild notochord deformities. The coronal and sagittal planes are reconstructed from µCT images of the fish in the upper panels, each showing a gross appearance. The red arrows indicate vertebral malformation. (F) Diagram of zebrafish growth stages. Continuous observation of one GATA2-1b:lbx1b-injected larva with a displaced dorsal melanophore stripe, but without notochord deformation, from 6 to 90 dpf. The red and green dotted lines indicate the dorsal middle lines and the right upper boundary of the lateral stripe, respectively. Progressive scoliosis with rotation of the longitudinal axis of the body was observed after 55 dpf. E, embryo; L, larva; J, juvenile; A, adult. (G) µCT analysis of the three-dimensional structure of the spine of the same zebrafish in (F) showing scoliosis. Cobb’s angle was measured in the coronal plane. Scale bars in (B): 200 µm; (C): 1 mm or 100 µm; (D): 500 µm; and (F): 1 mm. |
|
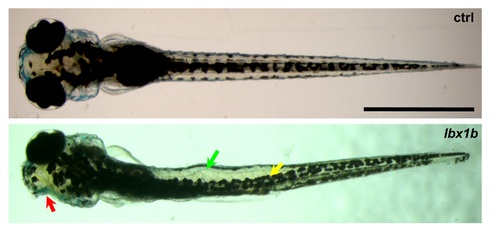
Local deformation of the notochord, displaced dorsal melanophore stripe, and ophthalmic abnormity caused by injection of lbx1b mRNA. Dorsal views of larvae at 6 dpf. The deformed notochord, displaced dorsal melanophore stripe, and anophthalmia in lbx1b mRNA-injected zebrafish are indicated by a green, yellow, and red arrow, respectively. Scale bar: 1 mm. |
|
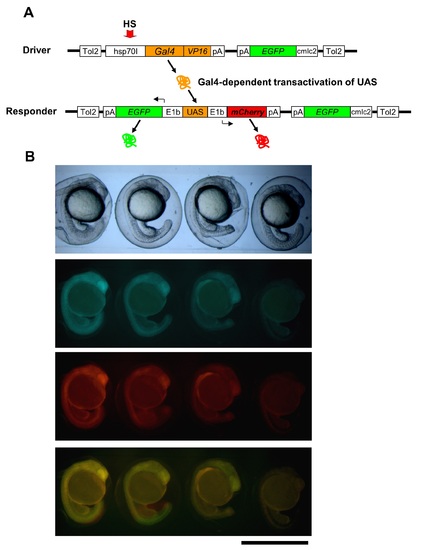
Gal4/UAS-based bidirectional expression system. (A) The constructs of the Gal4/UAS-based bidirectional expression system. Heat shock (HS) treatment activates the hsp70I promoter in the driver construct (Driver) to express Gal4-VP16. Gal4-VP16 protein binds to the UAS on the responder construct (Responder) and activates the expression of mCherry and EGFP via E1b minimal promoters. (B) A positive correlation of expression level between the two genes flanking the UAS in Tg(hsp:Gal4-VP: EGFP:UAS:mcherry). The scale bar represents 1 mm. |
|
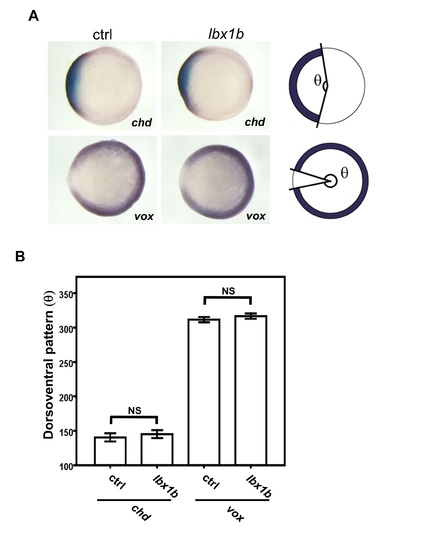
Normal dorsoventral patterning in embryos with lbx1b overexpression. (A) Whole-mount in situ hybridization (WISH) for dorsal organizer gene chd and ventral gene vox in shield-stage embryos injected with buffer as control (ctrl) or lbx1b mRNA (lbx1b). Photos are taken from the animal pole side of embryos with dorsal to the left. (B) Quantitative analysis of the WISH signals shown in A. For quantification of the dorsoventral position, the angle θ [degree] shown in the panel A as measured. No significant change was observed for both chd (p = 0.261) and vox (p = 0.071) expression pattern. The numbers of control and lbx1b zebrafish embryos were 22 and 23 for chd, 23 and 23 for vox, respectively. |
|
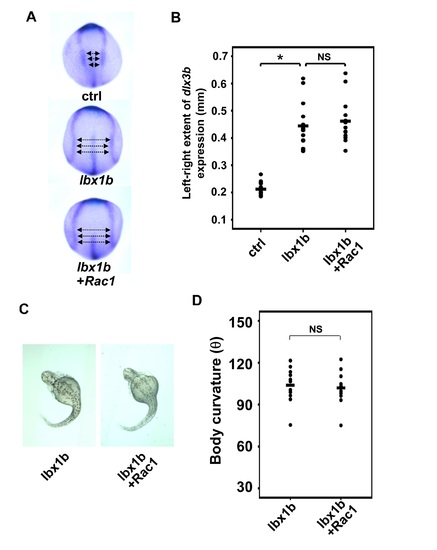
Failure to rescue defective convergent extension and body curvature in lbx1b-overexpressing embryos by Rac1 mRNA injection. (A) Dorsal view of dlx3b/hgg1/ntl expression in the tail bud of embryos injected with buffer (ctrl), lbx1b mRNA (lbx1b), or lbx1b and human RAC1 mRNA (lbx1b+Rac1). (B) Quantitative analysis of convergent extension (CE) movement with embryos in (A) (ctrl, n = 15; lbx1b, n = 15; lbx1b+Rac1, n = 13). The extent of defective CE was evaluated by measuring the distance between the inner edges of bilateral dlx3b expression at 3 regions as indicated by the arrows in panel A. RAC1 mRNA injection failed to rescue defective CE. *p < 0.01, NS: not significant. (C) Dorsal view of 48 hpf Tg(hsp:Gal-VP; EGFP:UAS:lbx1b) embryos upon heat shock at 4 hpf with buffer (lbx1b) or RAC1 mRNA (lbx1b+Rac1) injection. (D) Quantitative analysis of body curvature with the embryos in (C) (lbx1b, n = 11; lbx1b+Rac1, n = 11). No significant change of the severity of body curvature was observed in RAC1 mRNA-injected embryos. NS: not significant. Severity of body curvature was quantified by the angle as described in Fig 3. F2 lines of driver and responder transgenic fish were used. The dosages for embryo injections were 50 pg/embryo for lbx1b mRNA and 40 pg/embryo for RAC1 mRNA. |
|
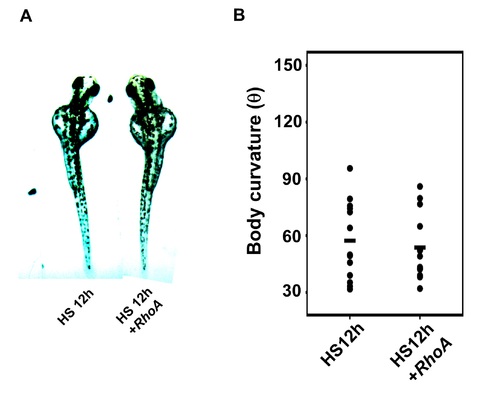
Effect of RhoA mRNA injection on body curvature of Tg(hsp:Gal-VP:EGFP:UAS:lbx1b) with heat shock at 12 hpf. (A) Dorsal views of 48 hpf Tg(hsp:Gal-VP:EGFP:UAS:lbx1b) embryos upon heat shock (HS) at 12 hpf with buffer (HS12h) or RhoA mRNA (HS12h+RhoA) injection. (B) Quantitative analysis of body curvature in Tg(hsp:Gal-VP:EGFP:UAS:lbx1b) embryos upon HS at 12 hpf with buffer injection (lbx1b, n = 13) or RhoA mRNA injection (lbx1b+RhoA, n = 13). Significant change in axis development was not observed in RhoA-injected embryos with HS at 12 hpf. Severity of body curvature was quantified by the angle as shown in Fig 3E. F2 lines of driver and responder transgenic fish were used. |
|
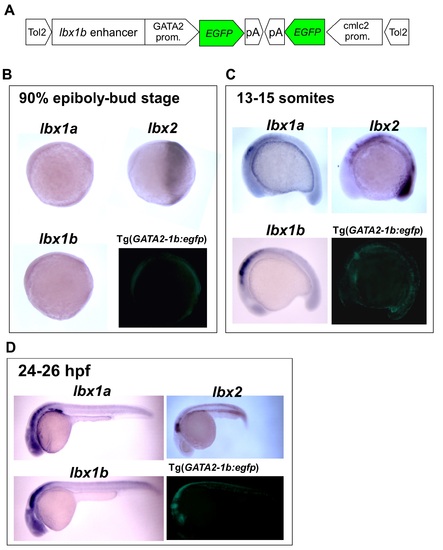
Characterization of an lbx1b enhancer in Tg(GATA2-1b:EGFP). (A) Construction of the transgene. The GATA2 minimal promoter and an lbx1b enhancer cooperatively drive the expression of EGFP. The cardiac specific promoter cmlc2 drives EGFP expression in the heart as a transgenic marker. (B-D) Comparison of EGFP fluorescence with lbx1a, lbx1b, and lbx2 expression in lateral views at the 90% epiboly-bud stage (B), 13-15 somites stage (C), and 24-26 somites stage (D). |
|
Body curvature in embryos with lbx1b expressed under the control of the GATA2 minimal promoter and an lbx1b enhancer. (A) Dorsal views of live embryos at 48 hpf. Body curvature was observed in zebrafish injected with GATA2-1b:lbx1b (lbx1b), but not in those injected with GATA2-1b:MCS (ctrl). The scale bar represents 500 µm. (B) Quantitative analysis of body curvature (cur) in 48 hpf embryos. The incidence of body curvature was significantly increased in lbx1b embryos (ctrl, 5%, n = 83; lbx1b, 46%, n = 94. p < 0.01). Scale bar in (A): 500 µm. |
|
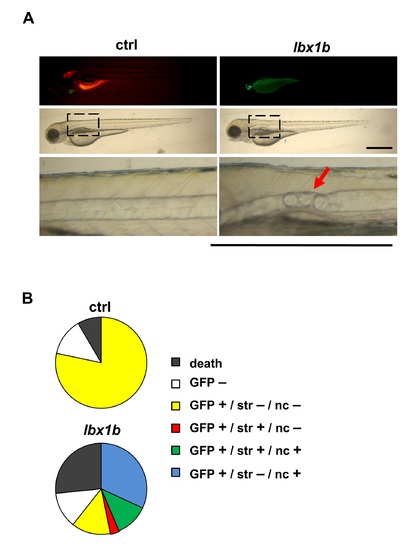
Notochord deformity and displaced dorsal melanophore stripe in embryos with lbx1b expressed under the control of the GATA2 minimal promoter and an lbx1b enhancer. (A) Lateral views of embryos (48 hpf) injected with GATA2-1b:mCherry (ctrl) or GATA2-1b:lbx1b (lbx1b). Local notochord deformation (red arrow) was observed in lbx1b embryos. The lower panels show magnified views of the areas indicated by the dotted boxes in the corresponding middle panels. Scale bars represent 500 µm. (B) Quantitative analysis of the phenotypes of notochord deformation (nc+) and displaced dorsal melanophore stripe (str+) in 6 dpf zebrafish (ctrl, n = 224; lbx1b, n = 288). |
|
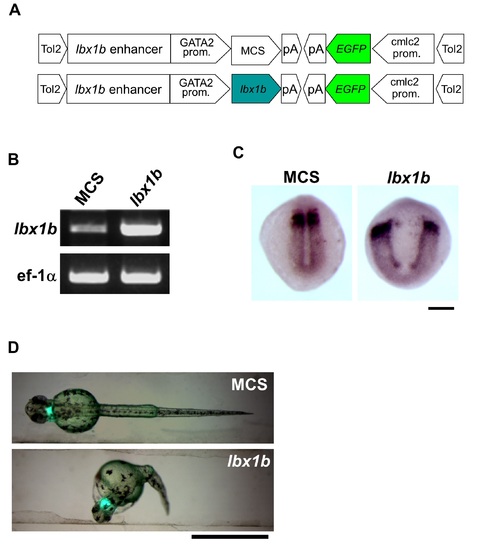
Severe convergent extension defects and body curvature in Tg(GATA2-1b:lbx1b) F1 embryos. (A) The constructs used for transgenesis in zebrafish with the tol2 transposon system are shown. The cardiac specific promoter cmlc2 drives EGFP expression in the heart as a transgenic marker. (B) RT-PCR for 11 hpf embryos of Tg(GATA2-1b:MCS) F1 (MCS) and Tg(GATA2-1b:lbx1b) F1 (lbx1b) shows the elevated expression of lbx1b in lbx1b embryos. Ef-1α was used as a constitutive control. (C) Dorsal views of In situ hybridization for papc (paraxial mesoderm marker) at 11 hpf. Severe convergent defects were found in lbx1b embryos. The scale bar represents 200 µm. (D) Dorsal views of embryos at 48 hpf. Severe body curvature was observed in lbx1b embryos. The scale bar represents 1 mm. |